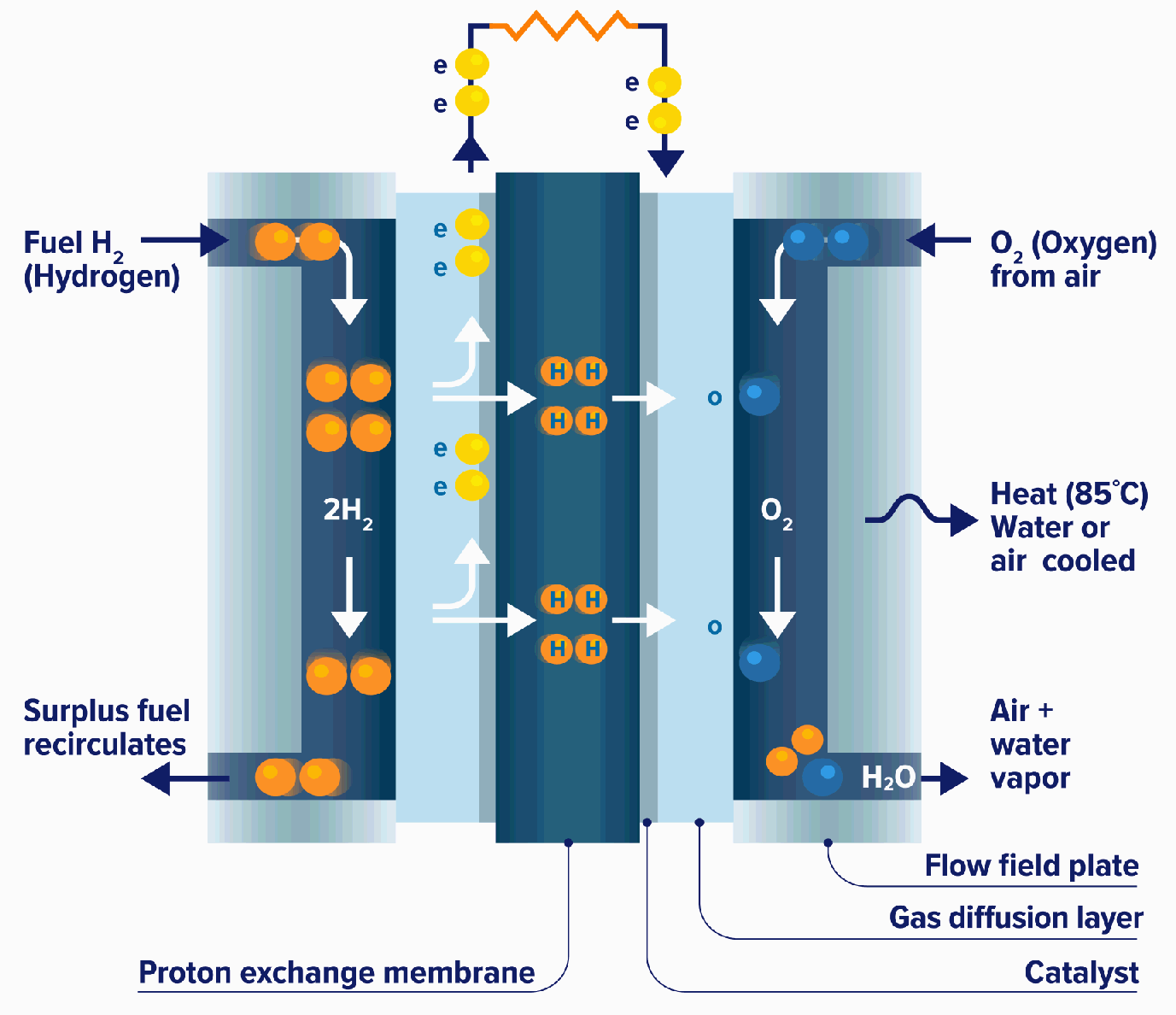
Fuel Cell Chemistry . They produce electricity and heat as long as fuel is supplied. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells work like batteries, but they do not run down or need recharging. What is a fuel cell? Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. A fuel cell is a device that converts chemical energy into electrical energy. Fuel cells have an important advantage. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Unlike a battery, it does not store. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell consists of two electrodes—a. Fuel cells are similar to batteries but. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions.
from fuelcellscars.com
A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. They produce electricity and heat as long as fuel is supplied. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells are similar to batteries but. Unlike a battery, it does not store. A fuel cell is a device that converts chemical energy into electrical energy. Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. Fuel cells work like batteries, but they do not run down or need recharging.
ALL ABOUT FUEL CELLS HOW DO THEY WORK
Fuel Cell Chemistry Fuel cells are similar to batteries but. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. What is a fuel cell? A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Unlike a battery, it does not store. A fuel cell consists of two electrodes—a. A fuel cell is a device that converts chemical energy into electrical energy. Fuel cells have an important advantage. Fuel cells are similar to batteries but. They produce electricity and heat as long as fuel is supplied. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells work like batteries, but they do not run down or need recharging. Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications.
From nl.mathworks.com
Fuel Cell Model MATLAB & Simulink Fuel Cell Chemistry Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. They produce electricity and heat as long as fuel is supplied. A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell like this will continue to operate and produce electrical energy as. Fuel Cell Chemistry.
From www.britannica.com
Fuel cell Definition, Types, Applications, & Facts Britannica Fuel Cell Chemistry It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell consists of two electrodes—a. A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel. Fuel Cell Chemistry.
From www.britannica.com
Fuel cell Proton Exchange, Alkaline, Polymer Britannica Fuel Cell Chemistry Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. What is a fuel cell? It is defined as an electrochemical cell that generates electrical energy. Fuel Cell Chemistry.
From www.savemyexams.com
Fuel Cells SL IB Chemistry Revision Notes 2025 Fuel Cell Chemistry They produce electricity and heat as long as fuel is supplied. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. What is a fuel cell? A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time.. Fuel Cell Chemistry.
From chemistry.stackexchange.com
electrochemistry Electrode polarity in fuel cells Chemistry Stack Fuel Cell Chemistry Fuel cells work like batteries, but they do not run down or need recharging. What is a fuel cell? Fuel cells have an important advantage. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell resembles a battery in many respects, but it can supply. Fuel Cell Chemistry.
From www.youtube.com
Direct Methanol Fuel Cell, Introduction, Principle, Advantages Fuel Cell Chemistry Fuel cells work like batteries, but they do not run down or need recharging. A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Fuel cells are similar to batteries but. Learn about. Fuel Cell Chemistry.
From www.tes.com
Electrolysis and Fuel Cells GCSE Chemistry Teaching Resources Fuel Cell Chemistry A fuel cell consists of two electrodes—a. Fuel cells have an important advantage. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Fuel cells work like batteries, but they do not run down or need recharging. It is defined as an electrochemical cell that generates. Fuel Cell Chemistry.
From 640orfree.com
How Does A Hydrogen Fuel Cell Work? A Comprehensive Guide Linquip (2022) Fuel Cell Chemistry They produce electricity and heat as long as fuel is supplied. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell consists of two electrodes—a. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen. Fuel Cell Chemistry.
From www.youtube.com
Fuel Cells for AQA GCSE Chemistry YouTube Fuel Cell Chemistry What is a fuel cell? They produce electricity and heat as long as fuel is supplied. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel. Fuel Cell Chemistry.
From pressbooks.openedmb.ca
Batteries and Fuel Cells Chemistry and the Environment Fuel Cell Chemistry A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. Fuel. Fuel Cell Chemistry.
From chem.libretexts.org
6.5 Batteries and Fuel Cells Chemistry LibreTexts Fuel Cell Chemistry A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. A fuel cell resembles a battery in many respects, but it can supply electrical. Fuel Cell Chemistry.
From www.slideserve.com
PPT Fuel Cell Design PowerPoint Presentation, free download ID4176552 Fuel Cell Chemistry What is a fuel cell? A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells have an important advantage. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. A fuel cell. Fuel Cell Chemistry.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry for Majors Fuel Cell Chemistry Unlike a battery, it does not store. They produce electricity and heat as long as fuel is supplied. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage. Fuel cells are similar to batteries but. A fuel cell is a. Fuel Cell Chemistry.
From www.engineeringity.com
Breaking Down Fuel Cells How they Work and their Components The Fuel Cell Chemistry It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. They produce electricity and heat as long as fuel is supplied. Fuel cells have an important advantage. A fuel cell is a. Fuel Cell Chemistry.
From guidewiringdietician.z5.web.core.windows.net
Fuel Cell Diagram Gcse Fuel Cell Chemistry Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. A fuel cell consists of two electrodes—a. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell resembles a battery in. Fuel Cell Chemistry.
From courses.lumenlearning.com
Batteries and Fuel Cells Chemistry Atoms First Fuel Cell Chemistry What is a fuel cell? A fuel cell consists of two electrodes—a. Fuel cells have an important advantage. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges. Fuel Cell Chemistry.
From fuelcellscars.com
ALL ABOUT FUEL CELLS HOW DO THEY WORK Fuel Cell Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells are similar to batteries but. Unlike a battery, it does not store. What is a fuel cell? A fuel cell consists of two electrodes—a. Learn about the basics of fuel cell chemistry, the types of fuel. Fuel Cell Chemistry.
From www.researchgate.net
Schematic representation of Direct Methanol Fuel Cell (DMFC). This fuel Fuel Cell Chemistry A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell consists of two electrodes—a. Fuel cells are similar to batteries but. Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. Fuel cell, any of a class of devices that convert the. Fuel Cell Chemistry.
From revisechemistry.uk
Chemical Cells and Fuel Cells AQA C5 revisechemistry.uk Fuel Cell Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. They produce electricity and heat as long as fuel is supplied. A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell like this will continue to operate and produce electrical energy as. Fuel Cell Chemistry.
From large.stanford.edu
Hydrogen Fuel Cells Fuel Cell Chemistry Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. They produce electricity and heat as long as fuel is supplied. What is a. Fuel Cell Chemistry.
From mungfali.com
Fuel Cell Anode And Cathode Fuel Cell Chemistry They produce electricity and heat as long as fuel is supplied. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells work like batteries, but they do not run down or need recharging. A fuel cell consists of two electrodes—a. Unlike a battery, it does not. Fuel Cell Chemistry.
From www.youtube.com
OCR C6 Fuel Cells (Higher) YouTube Fuel Cell Chemistry Fuel cells work like batteries, but they do not run down or need recharging. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are similar to batteries but. They produce electricity and heat as long as fuel is supplied. Learn about the basics of fuel. Fuel Cell Chemistry.
From www.chfca.ca
About Fuel Cells CHFCA Fuel Cell Chemistry A fuel cell is a device that converts chemical energy into electrical energy. What is a fuel cell? Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are similar to batteries but. Fuel cells have an important advantage. It is defined as an electrochemical cell. Fuel Cell Chemistry.
From courses.lumenlearning.com
17.5 Batteries and Fuel Cells Chemistry Fuel Cell Chemistry Unlike a battery, it does not store. Fuel cells work like batteries, but they do not run down or need recharging. They produce electricity and heat as long as fuel is supplied. Fuel cells are similar to batteries but. What is a fuel cell? Fuel cell, any of a class of devices that convert the chemical energy of a fuel. Fuel Cell Chemistry.
From philschatz.com
Batteries and Fuel Cells · Chemistry Fuel Cell Chemistry Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. They produce electricity and heat as long as fuel is supplied. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell is a. Fuel Cell Chemistry.
From isp.page
Understanding Hydrogen Fuel Cell Chemistry Fuel Cell Chemistry They produce electricity and heat as long as fuel is supplied. Fuel cells have an important advantage. Unlike a battery, it does not store. Fuel cells are similar to batteries but. A fuel cell consists of two electrodes—a. A fuel cell is a device that converts chemical energy into electrical energy. What is a fuel cell? A fuel cell is. Fuel Cell Chemistry.
From www.e-conversion.de
Fuel cell principle econversion Fuel Cell Chemistry Fuel cells are similar to batteries but. A fuel cell consists of two electrodes—a. Unlike a battery, it does not store. They produce electricity and heat as long as fuel is supplied. Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. A fuel cell like this will. Fuel Cell Chemistry.
From chem.libretexts.org
Case Study Fuel Cells Chemistry LibreTexts Fuel Cell Chemistry A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Unlike a battery, it does not store. Learn about the basics of fuel cell. Fuel Cell Chemistry.
From www.slideserve.com
PPT Fuel cells PowerPoint Presentation, free download ID1590103 Fuel Cell Chemistry A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Learn about the basics of fuel cell chemistry, the types of fuel cells, and the benefits and challenges of fuel cell applications. They produce electricity and heat as long as fuel is supplied. Fuel cell, any. Fuel Cell Chemistry.
From courses.lumenlearning.com
Phases and Classification of Matter Introductory Chemistry Lecture Fuel Cell Chemistry Fuel cells have an important advantage. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cells are similar to batteries but. What is a fuel cell? A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the. Fuel Cell Chemistry.
From www.youtube.com
fuel cells electrochemistry 2 class 12 chemistry subject notes lectures Fuel Cell Chemistry Unlike a battery, it does not store. They produce electricity and heat as long as fuel is supplied. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells work like batteries, but they do not run down or need recharging. A fuel cell is a device. Fuel Cell Chemistry.
From www.youtube.com
Fuel Cell O level / IGCSE Chemistry YouTube Fuel Cell Chemistry A fuel cell is a device that converts chemical energy into electrical energy. A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. Fuel cells are. Fuel Cell Chemistry.
From www.vectorstock.com
Molten carbonate fuel cells process Royalty Free Vector Fuel Cell Chemistry A fuel cell consists of two electrodes—a. Unlike a battery, it does not store. It is defined as an electrochemical cell that generates electrical energy from fuel via electrochemical reactions. A fuel cell is a galvanic cell that requires a constant external supply of reactants because the products of the reaction are continuously removed. Fuel cells have an important advantage.. Fuel Cell Chemistry.
From www.researchgate.net
Scheme of a solid oxide fuel cell. Download Scientific Diagram Fuel Cell Chemistry They produce electricity and heat as long as fuel is supplied. What is a fuel cell? A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. Fuel cells have an important advantage. It is defined as an electrochemical cell that generates electrical energy from fuel via. Fuel Cell Chemistry.
From mmerevise.co.uk
Electrochemical Cells Worksheets and Revision MME Fuel Cell Chemistry A fuel cell like this will continue to operate and produce electrical energy as long as a supply of hydrogen and oxygen are available. A fuel cell is a device that converts chemical energy into electrical energy. Fuel cell, any of a class of devices that convert the chemical energy of a fuel directly into electricity by electrochemical reactions. They. Fuel Cell Chemistry.