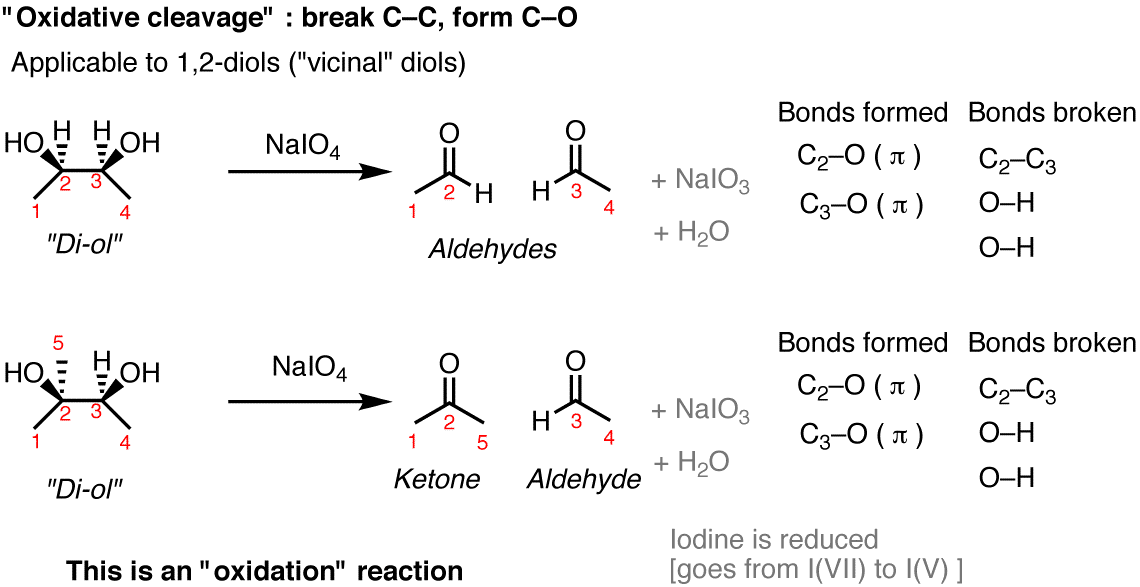
Draw The Products Formed In The Following Oxidative Cleavage . Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. The term oxidative cleavage itself defines the reaction. Here’s how to approach this question. Because at least one of the reaction products is a. Draw the products formed in each oxidative cleavage. Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or kmno 4, although the reaction is of little value. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. The author explains that the products formed in oxidative.
from www.masterorganicchemistry.com
The term oxidative cleavage itself defines the reaction. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Here’s how to approach this question. The author explains that the products formed in oxidative. Draw the products formed in each oxidative cleavage. Because at least one of the reaction products is a. Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or kmno 4, although the reaction is of little value. Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide.
Introduction to Oxidative Cleavage Reactions Master Organic Chemistry
Draw The Products Formed In The Following Oxidative Cleavage Here’s how to approach this question. The term oxidative cleavage itself defines the reaction. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Draw the products formed in each oxidative cleavage. The author explains that the products formed in oxidative. Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Here’s how to approach this question. Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or kmno 4, although the reaction is of little value. Because at least one of the reaction products is a. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide.
From www.masterorganicchemistry.com
Introduction to Oxidative Cleavage Reactions Master Organic Chemistry Draw The Products Formed In The Following Oxidative Cleavage Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. Because at least one of the reaction products is a. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Draw the correct product(s) for the following Draw The Products Formed In The Following Oxidative Cleavage The term oxidative cleavage itself defines the reaction. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. The author explains that the products formed in oxidative. During strong oxidation with ozone or. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Propose A Structure For The Hydrocarbon That Gives... Draw The Products Formed In The Following Oxidative Cleavage Here’s how to approach this question. The term oxidative cleavage itself defines the reaction. Because at least one of the reaction products is a. Draw the products formed in each oxidative cleavage. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. During strong oxidation with ozone. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chemistrysteps.com
Oxidative Cleavage of Alkenes with KMno4 and O3 Chemistry Steps Draw The Products Formed In The Following Oxidative Cleavage During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Here’s how to approach this question. The term oxidative cleavage itself defines the reaction. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. Identify the acetylide anion by examining the structure of the. Draw The Products Formed In The Following Oxidative Cleavage.
From www.masterorganicchemistry.com
Introduction to Oxidative Cleavage Reactions — Master Organic Chemistry Draw The Products Formed In The Following Oxidative Cleavage The term oxidative cleavage itself defines the reaction. Because at least one of the reaction products is a. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. Here’s how to approach this question. The author explains that the products formed in oxidative. Alkynes, like alkenes, can. Draw The Products Formed In The Following Oxidative Cleavage.
From www.numerade.com
SOLVED What alkyne (or diyne) yields the following oxidative cleavage products? Click the "draw Draw The Products Formed In The Following Oxidative Cleavage During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. Draw the products formed in each oxidative cleavage. The author explains that the products formed in oxidative. Ozonolysis is a method. Draw The Products Formed In The Following Oxidative Cleavage.
From www.masterorganicchemistry.com
Introduction to Oxidative Cleavage Reactions Master Organic Chemistry Draw The Products Formed In The Following Oxidative Cleavage Here’s how to approach this question. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. Because at least one of the reaction products is a. Alkynes, like alkenes, can be cleaved by reaction. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Sec. Ex. 19a Oxidative Cleavage of an Alkene by Draw The Products Formed In The Following Oxidative Cleavage Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. The author explains that the products formed in oxidative. Write an equation to describe the cleavage of an alkene by ozone, followed by. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved The product of the following oxidative cleavage with Draw The Products Formed In The Following Oxidative Cleavage Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or kmno 4, although the reaction is of little value. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation. Draw The Products Formed In The Following Oxidative Cleavage.
From www.numerade.com
SOLVED Draw the products formed in the following oxidative cleavage [1] O3 [2] H2O Draw Draw The Products Formed In The Following Oxidative Cleavage The author explains that the products formed in oxidative. Because at least one of the reaction products is a. Here’s how to approach this question. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so. Draw The Products Formed In The Following Oxidative Cleavage.
From www.numerade.com
Show the structures of alkenes that give the following products on oxidative cleavage with KMnO4 Draw The Products Formed In The Following Oxidative Cleavage During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Draw the products formed in each oxidative cleavage. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Draw the correct product(s) for the oxidative Draw The Products Formed In The Following Oxidative Cleavage Draw the products formed in each oxidative cleavage. Because at least one of the reaction products is a. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. The author explains that the products formed in oxidative. Alkynes, like alkenes, can be cleaved by reaction with powerful. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Draw the correct product(s) for the following Draw The Products Formed In The Following Oxidative Cleavage Here’s how to approach this question. Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or kmno 4, although the reaction is of little value. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. Write an equation to describe the cleavage of an. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Consider the following oxidative cleavage reactions Draw The Products Formed In The Following Oxidative Cleavage The author explains that the products formed in oxidative. Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Here’s how to approach this question. Because at least one of the reaction products is a. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Draw the two possible products of this oxidative Draw The Products Formed In The Following Oxidative Cleavage Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or kmno 4, although the reaction is of little value. The author explains that the products formed in oxidative. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. Write an equation to describe the. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved What alkyne (or dine) yields the following oxidative Draw The Products Formed In The Following Oxidative Cleavage Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. The term oxidative cleavage itself defines the reaction. Because at least one of the reaction products is a. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. The author explains that the. Draw The Products Formed In The Following Oxidative Cleavage.
From www.numerade.com
SOLVED Draw the correct product(s) for the oxidative cleavage reaction; Select Draw Rings More Draw The Products Formed In The Following Oxidative Cleavage Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. The author explains that the products formed in oxidative. Draw the products formed in each oxidative cleavage. Alkynes, like alkenes, can be cleaved by reaction with powerful. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved 7. Draw the products formed in each oxidative Draw The Products Formed In The Following Oxidative Cleavage The author explains that the products formed in oxidative. Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or kmno 4, although the reaction is of little value. The term oxidative cleavage itself defines the reaction. Here’s. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Each of the following involves an oxidative cleavage Draw The Products Formed In The Following Oxidative Cleavage Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen.. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Draw the products formed in the following oxidative Draw The Products Formed In The Following Oxidative Cleavage Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. The author explains that the products formed in oxidative. The term oxidative cleavage itself defines the reaction. Here’s how to approach. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Draw the structure for the alkene that yields the Draw The Products Formed In The Following Oxidative Cleavage Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. The term oxidative cleavage itself defines the reaction. Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Here’s how to approach this question. Because at least one of the reaction products is. Draw The Products Formed In The Following Oxidative Cleavage.
From www.numerade.com
SOLVED Propose structure for the hydrocarbon that gives the following products oxidative Draw The Products Formed In The Following Oxidative Cleavage Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Because at least one of the reaction products is a. Here’s how to approach this question. Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or kmno 4, although the reaction is of little value. During strong. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Draw the correct product(s) for the following Draw The Products Formed In The Following Oxidative Cleavage The term oxidative cleavage itself defines the reaction. Draw the products formed in each oxidative cleavage. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Alkynes, like alkenes, can. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Draw the correct products, in either order, for the Draw The Products Formed In The Following Oxidative Cleavage Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. The author explains that the products formed in oxidative. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Draw the two possible products of this oxidative Draw The Products Formed In The Following Oxidative Cleavage Here’s how to approach this question. The author explains that the products formed in oxidative. Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or kmno 4, although the reaction is of little value. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed. Draw The Products Formed In The Following Oxidative Cleavage.
From www.masterorganicchemistry.com
Introduction to Oxidative Cleavage Reactions Master Organic Chemistry Draw The Products Formed In The Following Oxidative Cleavage Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Because at least one of the reaction products is a. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Draw the products formed in each oxidative cleavage. Write an equation to describe the cleavage of an. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Question 3. the following oxidative Draw The Products Formed In The Following Oxidative Cleavage Because at least one of the reaction products is a. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. The author explains that the products formed in oxidative. Draw the products formed in each oxidative cleavage. Identify the acetylide anion by examining the structure of the. Draw The Products Formed In The Following Oxidative Cleavage.
From writersabc.com
Oxidative Cleavage Reaction. WritersABC Draw The Products Formed In The Following Oxidative Cleavage Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. Here’s how to approach this question. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. Draw the products formed in each oxidative cleavage. Identify the acetylide. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Draw the correct product(s) for the following Draw The Products Formed In The Following Oxidative Cleavage During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Draw the products formed in each oxidative cleavage. Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved What alkene or alkyne yields the following products Draw The Products Formed In The Following Oxidative Cleavage During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Draw the products formed in each oxidative cleavage. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. Because at least one of the reaction products is a. The author explains. Draw The Products Formed In The Following Oxidative Cleavage.
From www.numerade.com
SOLVED Draw the alkene oxidative starting cleavage material and products. oxidizing Products Draw The Products Formed In The Following Oxidative Cleavage Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so formed with hydrogen peroxide. Here’s how to approach this question. Draw the products formed in each oxidative cleavage. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive allotrope of oxygen. During strong oxidation. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Draw the correct products, in either order, for the Draw The Products Formed In The Following Oxidative Cleavage Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or kmno 4, although the reaction is of little value. Here’s how to approach this question. Because at least one of the reaction products is a. Write an equation to describe the cleavage of an alkene by ozone, followed by oxidation of the ozonide so. Draw The Products Formed In The Following Oxidative Cleavage.
From www.chegg.com
Solved Consider the following oxidative cleavage reactions Draw The Products Formed In The Following Oxidative Cleavage During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Draw the products formed in each oxidative cleavage. Alkynes, like alkenes, can be cleaved by reaction with powerful oxidizing agents such as ozone or kmno 4, although the reaction is of little value. Write an equation to describe the cleavage of an alkene by. Draw The Products Formed In The Following Oxidative Cleavage.
From www.numerade.com
SOLVED What alkene or alkyne yields the following products after oxidative cleavage with ozone Draw The Products Formed In The Following Oxidative Cleavage Identify the acetylide anion by examining the structure of the alkyne and removing the appropriate hydrogen atom. Because at least one of the reaction products is a. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive. Draw The Products Formed In The Following Oxidative Cleavage.
From quizlet.com
Draw the lower and higher mass product of the oxidative clea Quizlet Draw The Products Formed In The Following Oxidative Cleavage The author explains that the products formed in oxidative. During strong oxidation with ozone or basic potassium permanganate, the alkyne is cleaved into two products. Because at least one of the reaction products is a. Draw the products formed in each oxidative cleavage. Ozonolysis is a method of oxidatively cleaving alkenes or alkynes using ozone (o3 o 3), a reactive. Draw The Products Formed In The Following Oxidative Cleavage.