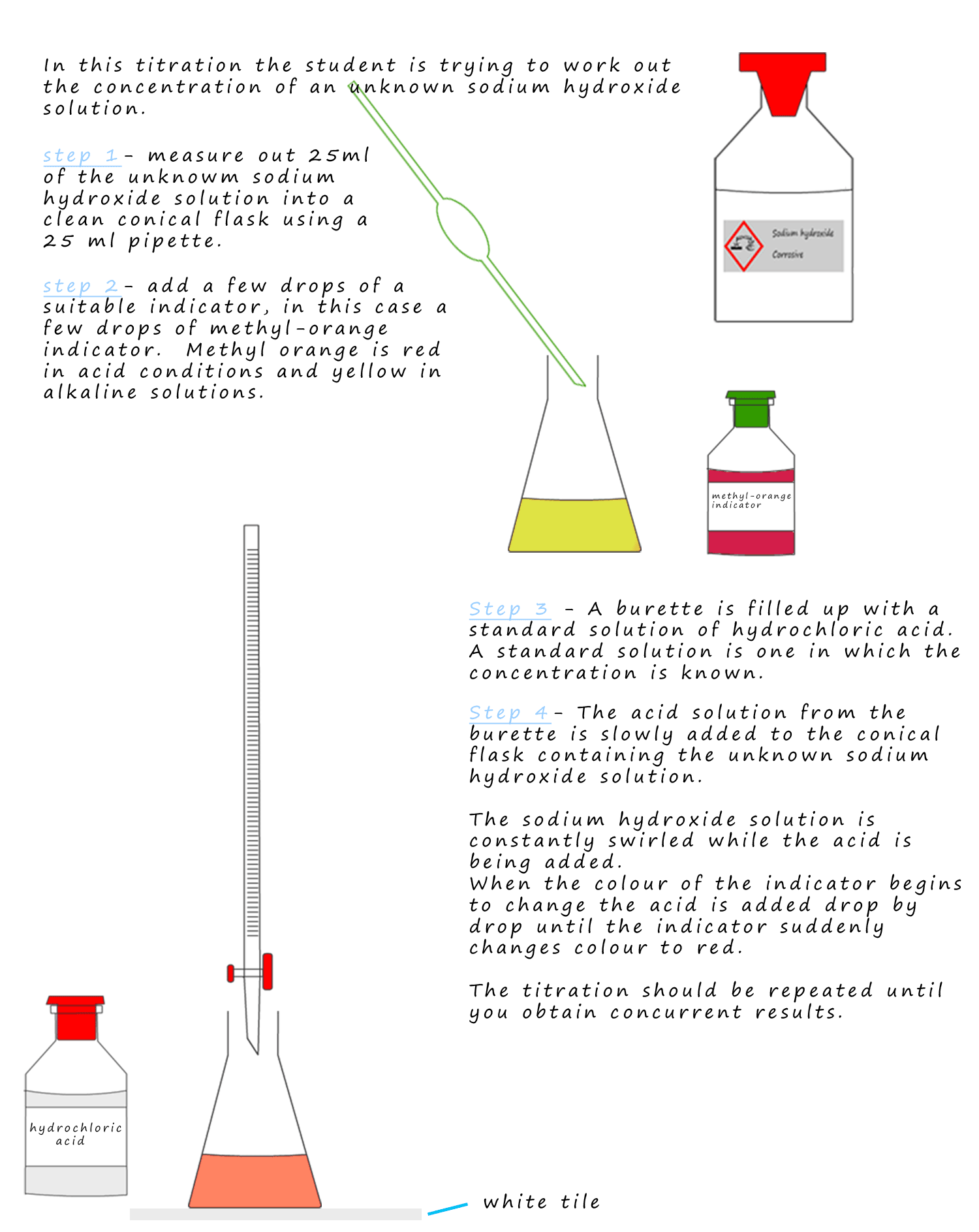
Standard Solution And Titration . A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. What is a standard solution? If the unknown solution is basic then the standard solution will be. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration requires a solution of accurately known concentration called a standard solution. Volumetric analysis is a process that uses the volume and concentration of one chemical.
from www.science-revision.co.uk
What is a standard solution? If the unknown solution is basic then the standard solution will be. Volumetric analysis is a process that uses the volume and concentration of one chemical. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. Titration requires a solution of accurately known concentration called a standard solution.
Titrations
Standard Solution And Titration If the unknown solution is basic then the standard solution will be. A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Volumetric analysis is a process that uses the volume and concentration of one chemical. If the unknown solution is basic then the standard solution will be. What is a standard solution? Titration requires a solution of accurately known concentration called a standard solution.
From www.slideserve.com
PPT Sodium Thiosulfate Titrations PowerPoint Presentation, free download ID2775939 Standard Solution And Titration What is a standard solution? A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. Volumetric analysis is a process that uses the volume and concentration of one chemical. Titration requires a solution of accurately known concentration called a. Standard Solution And Titration.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Standard Solution And Titration If the unknown solution is basic then the standard solution will be. Titration requires a solution of accurately known concentration called a standard solution. A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. Volumetric analysis is a process. Standard Solution And Titration.
From bramblechemistry.weebly.com
4C6 Titration Standard Solution And Titration If the unknown solution is basic then the standard solution will be. A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. Titration requires a solution of accurately known concentration called a standard solution. What is a standard solution?. Standard Solution And Titration.
From slideplayer.com
Titration Objectives ppt download Standard Solution And Titration Titration requires a solution of accurately known concentration called a standard solution. If the unknown solution is basic then the standard solution will be. What is a standard solution? Volumetric analysis is a process that uses the volume and concentration of one chemical. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Standard Solution And Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Standard Solution And Titration Volumetric analysis is a process that uses the volume and concentration of one chemical. Titration requires a solution of accurately known concentration called a standard solution. What is a standard solution? A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and. Standard Solution And Titration.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Point Standard Solution And Titration A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. If the unknown solution is basic then the standard solution will be. Titration requires a solution of accurately known concentration called a standard solution. What is a standard solution?. Standard Solution And Titration.
From www.chemicals.co.uk
Examples of Standard Solutions The Chemistry Blog Standard Solution And Titration What is a standard solution? A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. If the unknown solution. Standard Solution And Titration.
From mssmithsechs.weebly.com
Acids, Bases, Solutions, and Titrations MS. SMITH'S CLASS Standard Solution And Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Volumetric analysis is a process that uses the volume and concentration of one chemical. If the unknown solution is basic then the standard solution will be. What is a standard solution? A standard solution is a a solution of accurately. Standard Solution And Titration.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2145164 Standard Solution And Titration If the unknown solution is basic then the standard solution will be. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration requires a solution of accurately known concentration called a standard solution. A standard solution is a a solution of accurately known concentration prepared from a primary standard. Standard Solution And Titration.
From www.youtube.com
Standard Solution and Titration (BWD21303 Practical 2) YouTube Standard Solution And Titration Volumetric analysis is a process that uses the volume and concentration of one chemical. If the unknown solution is basic then the standard solution will be. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What is a standard solution? Titration requires a solution of accurately known concentration called. Standard Solution And Titration.
From www.science-revision.co.uk
Titrations Standard Solution And Titration Volumetric analysis is a process that uses the volume and concentration of one chemical. Titration requires a solution of accurately known concentration called a standard solution. If the unknown solution is basic then the standard solution will be. A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of. Standard Solution And Titration.
From azwikihow.blogspot.com
PREPARATION OF STANDARD SOLUTIONS (TITRATION) AZ WIKI HOW Standard Solution And Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. If the unknown solution is basic then the standard solution will be. Titration requires a solution of accurately known concentration called a standard solution. Volumetric analysis is a process that uses the volume and concentration of one chemical. What is. Standard Solution And Titration.
From quizlet.com
Titration and making a standard solution Required practical 1 Diagram Quizlet Standard Solution And Titration If the unknown solution is basic then the standard solution will be. Volumetric analysis is a process that uses the volume and concentration of one chemical. What is a standard solution? Titration requires a solution of accurately known concentration called a standard solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using. Standard Solution And Titration.
From www.youtube.com
28 TITRATION 3 Standard Solution HSC Chemistry YouTube Standard Solution And Titration Volumetric analysis is a process that uses the volume and concentration of one chemical. If the unknown solution is basic then the standard solution will be. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration requires a solution of accurately known concentration called a standard solution. What is. Standard Solution And Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Standard Solution And Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration requires a solution of accurately known concentration called a standard solution. What is a standard solution? If the unknown solution is basic then the standard solution will be. A standard solution is a a solution of accurately known concentration. Standard Solution And Titration.
From quiztrailingly.z21.web.core.windows.net
Primary Standard In Titration Standard Solution And Titration If the unknown solution is basic then the standard solution will be. Volumetric analysis is a process that uses the volume and concentration of one chemical. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What is a standard solution? Titration requires a solution of accurately known concentration called. Standard Solution And Titration.
From general.chemistrysteps.com
AcidBase Titrations Chemistry Steps Standard Solution And Titration A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. What is a standard solution? A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Volumetric analysis is a. Standard Solution And Titration.
From www.reagent.co.uk
How is Titration Used in the Pharmaceutical Industry? Standard Solution And Titration Volumetric analysis is a process that uses the volume and concentration of one chemical. Titration requires a solution of accurately known concentration called a standard solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. If the unknown solution is basic then the standard solution will be. What is. Standard Solution And Titration.
From www.tutormyself.com
(f) Acids, alkalis and titrations Archives TutorMyself Chemistry Standard Solution And Titration Volumetric analysis is a process that uses the volume and concentration of one chemical. Titration requires a solution of accurately known concentration called a standard solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A standard solution is a a solution of accurately known concentration prepared from a. Standard Solution And Titration.
From www.sciencephoto.com
Titration experiment Stock Image C004/7696 Science Photo Library Standard Solution And Titration A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. If the unknown solution is basic then the standard solution will be. Titration requires a solution of accurately known concentration called a standard solution. What is a standard solution?. Standard Solution And Titration.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Standard Solution And Titration If the unknown solution is basic then the standard solution will be. Volumetric analysis is a process that uses the volume and concentration of one chemical. What is a standard solution? A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A standard solution is a a solution of accurately. Standard Solution And Titration.
From www.youtube.com
Preparing standard solution and titration YouTube Standard Solution And Titration Volumetric analysis is a process that uses the volume and concentration of one chemical. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What is a standard solution? Titration requires a solution of accurately known concentration called a standard solution. If the unknown solution is basic then the standard. Standard Solution And Titration.
From www.chemicals.co.uk
What Is A Standard Solution? The Chemistry Blog Standard Solution And Titration Volumetric analysis is a process that uses the volume and concentration of one chemical. If the unknown solution is basic then the standard solution will be. A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. A titration is. Standard Solution And Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Standard Solution And Titration Titration requires a solution of accurately known concentration called a standard solution. A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. Volumetric analysis is a process that uses the volume and concentration of one chemical. A titration is. Standard Solution And Titration.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Point Standard Solution And Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. If the unknown solution is basic then the standard solution will be. What is a standard solution? Titration requires a solution of accurately known concentration called a standard solution. Volumetric analysis is a process that uses the volume and concentration. Standard Solution And Titration.
From www.youtube.com
primary and secondary standard solutiontitration practicalchristianity practical jee neet Standard Solution And Titration Titration requires a solution of accurately known concentration called a standard solution. If the unknown solution is basic then the standard solution will be. What is a standard solution? A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a.. Standard Solution And Titration.
From spmchemistry.blog.onlinetuition.com.my
Preparing Standard Solutions SPM Chemistry Standard Solution And Titration Titration requires a solution of accurately known concentration called a standard solution. What is a standard solution? A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. If the unknown solution is basic then the standard solution will be.. Standard Solution And Titration.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记1.7.2 Titrations翰林国际教育 Standard Solution And Titration If the unknown solution is basic then the standard solution will be. Titration requires a solution of accurately known concentration called a standard solution. What is a standard solution? A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Volumetric analysis is a process that uses the volume and concentration. Standard Solution And Titration.
From scienceready.com.au
Titration Standard Solution, Washing, Setup HSC Chemistry Science Ready Standard Solution And Titration What is a standard solution? Titration requires a solution of accurately known concentration called a standard solution. A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. Volumetric analysis is a process that uses the volume and concentration of. Standard Solution And Titration.
From sciencenotes.org
What Is a Primary Standard in Chemistry? Standard Solution And Titration Volumetric analysis is a process that uses the volume and concentration of one chemical. What is a standard solution? A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. If the unknown solution is basic then the standard solution. Standard Solution And Titration.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation ID4401864 Standard Solution And Titration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. If the unknown solution is basic then the standard. Standard Solution And Titration.
From wisc.pb.unizin.org
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104 Resource Book Standard Solution And Titration A standard solution is a a solution of accurately known concentration prepared from a primary standard (a compound which is stable, of high purity, highly soluble in water and of a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Volumetric analysis is a process that uses the volume. Standard Solution And Titration.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher Standard Solution And Titration If the unknown solution is basic then the standard solution will be. What is a standard solution? A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration requires a solution of accurately known concentration called a standard solution. A standard solution is a a solution of accurately known concentration. Standard Solution And Titration.
From scienceready.com.au
Titration Standard Solution, Washing, Setup HSC Chemistry Science Ready Standard Solution And Titration Titration requires a solution of accurately known concentration called a standard solution. Volumetric analysis is a process that uses the volume and concentration of one chemical. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. If the unknown solution is basic then the standard solution will be. A standard. Standard Solution And Titration.
From www.studocu.com
Titration and standard solution Sammy Joujou 12H Miss Lo Titrations and Standard Solutions How Standard Solution And Titration If the unknown solution is basic then the standard solution will be. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Volumetric analysis is a process that uses the volume and concentration of one chemical. Titration requires a solution of accurately known concentration called a standard solution. What is. Standard Solution And Titration.