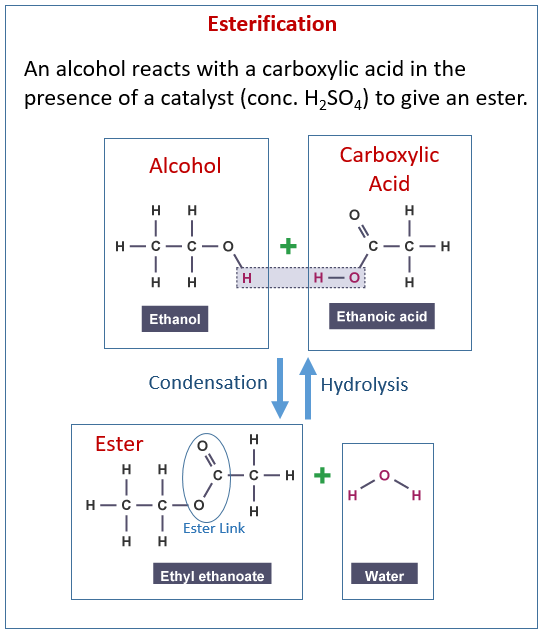
Why Is Esterification Important . The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most versatile chemical modifications of. Esterification is a chemical reaction in which two reactants such as alcohol and acid combine to create an ester as the reaction product. Esters are frequently the source of flavors and aromas in many fruits and flowers. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated sulphuric acid. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). The mechanism of ester reduction is similar to that of acid chloride reduction in that a.
from www.onlinemathlearning.com
Esterification is a chemical reaction in which two reactants such as alcohol and acid combine to create an ester as the reaction product. Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). Esters are frequently the source of flavors and aromas in many fruits and flowers. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most versatile chemical modifications of. The catalyst is usually concentrated sulphuric acid. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The mechanism of ester reduction is similar to that of acid chloride reduction in that a. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4).
Esters (examples, answers, activities, experiment, videos)
Why Is Esterification Important Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most versatile chemical modifications of. The catalyst is usually concentrated sulphuric acid. Esterification is a chemical reaction in which two reactants such as alcohol and acid combine to create an ester as the reaction product. The mechanism of ester reduction is similar to that of acid chloride reduction in that a. Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Esters are frequently the source of flavors and aromas in many fruits and flowers. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the.
From www.youtube.com
Esterification Reaction Organic Chemistry YouTube Why Is Esterification Important Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Esters are frequently the source of flavors and aromas in many fruits and flowers. Esterification is a chemical reaction in which two reactants such as alcohol and acid combine to create an ester as the reaction product. The mechanism of ester reduction is. Why Is Esterification Important.
From byjus.com
Why in esterification reaction oh part is removed from carboxylic acid Why Is Esterification Important The catalyst is usually concentrated sulphuric acid. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The π bond of the carbonyl group can act as a base to a. Why Is Esterification Important.
From www.coursehero.com
[Solved] . Explain why a base catalyzed esterification (shown below)... Course Hero Why Is Esterification Important 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most versatile chemical modifications of. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The π bond of the carbonyl group can act as a base to a strong inorganic acid due. Why Is Esterification Important.
From cookkim.com
Why Is Fischer Esterification Slow Unraveling The Chemistry Behind The Sluggish Reaction Why Is Esterification Important Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). The catalyst is usually concentrated sulphuric acid. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The mechanism of ester reduction is similar to that of acid chloride reduction in that a. Esterification is a. Why Is Esterification Important.
From www.slideserve.com
PPT Esters PowerPoint Presentation, free download ID2192161 Why Is Esterification Important The mechanism of ester reduction is similar to that of acid chloride reduction in that a. The catalyst is usually concentrated sulphuric acid. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Esterification is a chemical reaction in which two reactants such as alcohol and acid combine to create an ester as. Why Is Esterification Important.
From www.youtube.com
Esterification Process reaction mechanism Advance Organic Chemistry YouTube Why Is Esterification Important The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). The mechanism of ester reduction is similar to that of acid chloride reduction in that a. Esters are produced when carboxylic acids. Why Is Esterification Important.
From www.youtube.com
Esterification Important Mechanism The Point Of Chemistry YouTube Why Is Esterification Important The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Esterification is a chemical reaction in which two reactants such as alcohol and acid combine. Why Is Esterification Important.
From cookkim.com
Why Is Fischer Esterification Slow Unraveling The Chemistry Behind The Sluggish Reaction Why Is Esterification Important The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. The catalyst is usually concentrated sulphuric acid. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). Esters are frequently the source of flavors and aromas in many fruits and flowers. Esterification. Why Is Esterification Important.
From testbook.com
Esterification Learn Definition, Mechanism, Methods and Uses Why Is Esterification Important Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). The mechanism of ester reduction is similar to that of acid chloride reduction in that a. The catalyst is usually concentrated sulphuric acid. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Esterification is a. Why Is Esterification Important.
From nrochemistry.com
Fischer Esterification Why Is Esterification Important Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most versatile chemical modifications of. The catalyst is usually concentrated sulphuric acid. Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). Fischer. Why Is Esterification Important.
From cookkim.com
Why Is Fischer Esterification Slow Unraveling The Chemistry Behind The Sluggish Reaction Why Is Esterification Important Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most versatile chemical modifications of. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the.. Why Is Esterification Important.
From facts.net
18 Surprising Facts About Esterification Why Is Esterification Important The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a. Why Is Esterification Important.
From www.slideserve.com
PPT ESTERS PowerPoint Presentation, free download ID2246290 Why Is Esterification Important The mechanism of ester reduction is similar to that of acid chloride reduction in that a. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). The catalyst is usually concentrated sulphuric acid. Esterification is a chemical reaction in which two reactants such as alcohol and acid combine to create an ester as the reaction. Why Is Esterification Important.
From www.studypool.com
SOLUTION Esterification mechanism applications and importance in various industries Studypool Why Is Esterification Important 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most versatile chemical modifications of. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). Fischer esterification is the esterification of a carboxylic. Why Is Esterification Important.
From www.slideserve.com
PPT C7 Further Chemistry PowerPoint Presentation, free download ID2220026 Why Is Esterification Important Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Esters are frequently the source of flavors and aromas in many. Why Is Esterification Important.
From www.youtube.com
Esterification Reaction Formation of Ester Organic Chemistry YouTube Why Is Esterification Important The mechanism of ester reduction is similar to that of acid chloride reduction in that a. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). 19 rows esterification reaction can produce a broad spectrum of. Why Is Esterification Important.
From www.researchgate.net
3. Esterification of Fatty Acid. The diagram above illustrates an... Download Scientific Diagram Why Is Esterification Important Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most versatile chemical modifications of. The π bond of the carbonyl group can. Why Is Esterification Important.
From www.slideserve.com
PPT Esters and Esterification PowerPoint Presentation, free download ID4845736 Why Is Esterification Important Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). Esterification is a chemical reaction in which two reactants such as alcohol and acid combine to create an ester as the reaction product. The mechanism of ester reduction is similar to that of acid chloride reduction in that a. Fischer esterification is the esterification of. Why Is Esterification Important.
From www.numerade.com
SOLVED Explain why knowing the equilibrium constant for an esterification reaction is reaction Why Is Esterification Important Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). The catalyst is usually concentrated sulphuric acid. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most. Why Is Esterification Important.
From www.researchgate.net
Fischer Esterification (A) general esterification reaction by the... Download Scientific Diagram Why Is Esterification Important 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most versatile chemical modifications of. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The mechanism of ester reduction is similar to that of acid chloride reduction in that a. The catalyst is usually concentrated sulphuric acid.. Why Is Esterification Important.
From scienceinfo.com
Fischer Esterification Reaction Mechanism, Limitations Why Is Esterification Important Esterification is a chemical reaction in which two reactants such as alcohol and acid combine to create an ester as the reaction product. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The π bond of the carbonyl group can act as a base to a strong. Why Is Esterification Important.
From www.numerade.com
SOLVED In a a fischer esterification reaction Why is it important to neutralize the reaction Why Is Esterification Important Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The mechanism of ester reduction is similar to that of acid chloride reduction in that a. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of. Why Is Esterification Important.
From www.animalia-life.club
Esterification Mechanism Why Is Esterification Important Esterification is a chemical reaction in which two reactants such as alcohol and acid combine to create an ester as the reaction product. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. The catalyst is usually concentrated sulphuric acid. Esters are frequently the source of flavors. Why Is Esterification Important.
From what-is-this.net
ester définition What is Why Is Esterification Important The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. Esters are frequently the source of flavors and aromas in many fruits and flowers. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. Esterification. Why Is Esterification Important.
From studylib.net
esterification Why Is Esterification Important 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most versatile chemical modifications of. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Esterification is a chemical reaction in which two reactants such as alcohol and acid combine to create an ester as the reaction product.. Why Is Esterification Important.
From www.youtube.com
"ESTERIFICATION" explained BY V. A. SHANKAR YouTube Why Is Esterification Important The mechanism of ester reduction is similar to that of acid chloride reduction in that a. Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. Esters are frequently the. Why Is Esterification Important.
From www.chemistrysteps.com
Ester Hydrolysis Acid and BaseCatalyzed Mechanism Chemistry Steps Why Is Esterification Important Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in the presence of a strong acid. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. Esters are frequently the source of flavors and aromas in many fruits and flowers. Esters. Why Is Esterification Important.
From www.slideserve.com
PPT Esters and Esterification PowerPoint Presentation, free download ID4845736 Why Is Esterification Important Esterification is a chemical reaction in which two reactants such as alcohol and acid combine to create an ester as the reaction product. The catalyst is usually concentrated sulphuric acid. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). The π bond of the carbonyl group can act as a base to a strong. Why Is Esterification Important.
From www.onlinemathlearning.com
Esters (examples, answers, activities, experiment, videos) Why Is Esterification Important The catalyst is usually concentrated sulphuric acid. The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. Esters are frequently the source of flavors and aromas in many fruits and flowers. Fischer esterification is the esterification of a carboxylic acid by heating it with an alcohol in. Why Is Esterification Important.
From www.chemistrylearner.com
Fischer Esterification Definition, Examples, and Mechanism Why Is Esterification Important The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. Esters are frequently the source of flavors and aromas in many fruits and flowers. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). The mechanism of ester reduction is similar to. Why Is Esterification Important.
From www.slideserve.com
PPT Esterification reactions PowerPoint Presentation, free download ID293192 Why Is Esterification Important Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). The π bond of the carbonyl group can act as a base to a strong inorganic acid due to the distortion of the. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The mechanism of ester reduction. Why Is Esterification Important.
From testbook.com
Esterification Learn Definition, Mechanism, Methods and Uses Why Is Esterification Important Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). The catalyst is usually concentrated sulphuric acid. The mechanism of ester reduction is similar to that of acid chloride reduction in that a. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). Esters are produced when carboxylic. Why Is Esterification Important.
From www.coursehero.com
[Solved] Give the full mechanism for the process of an esterification... Course Hero Why Is Esterification Important Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). The mechanism of ester reduction is similar to that of acid chloride reduction in that a. The catalyst is usually concentrated sulphuric acid. Fischer esterification is the esterification. Why Is Esterification Important.
From testbook.com
Esterification Learn Definition, Mechanism, Methods and Uses Why Is Esterification Important Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Esters also make up the bulk of animal fats and vegetable oils—glycerides (fatty acid esters of glycerol). The catalyst is usually concentrated sulphuric acid. Esterification is a chemical. Why Is Esterification Important.
From studylib.net
Chemistry 209 Expt 5 Esterification Why Is Esterification Important 19 rows esterification reaction can produce a broad spectrum of bioproducts and, hence, is the most versatile chemical modifications of. Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. Esters are easily reduced by treatment with lialh 4 to yield primary alcohols (section 17.4). Esters are frequently the source of flavors and. Why Is Esterification Important.