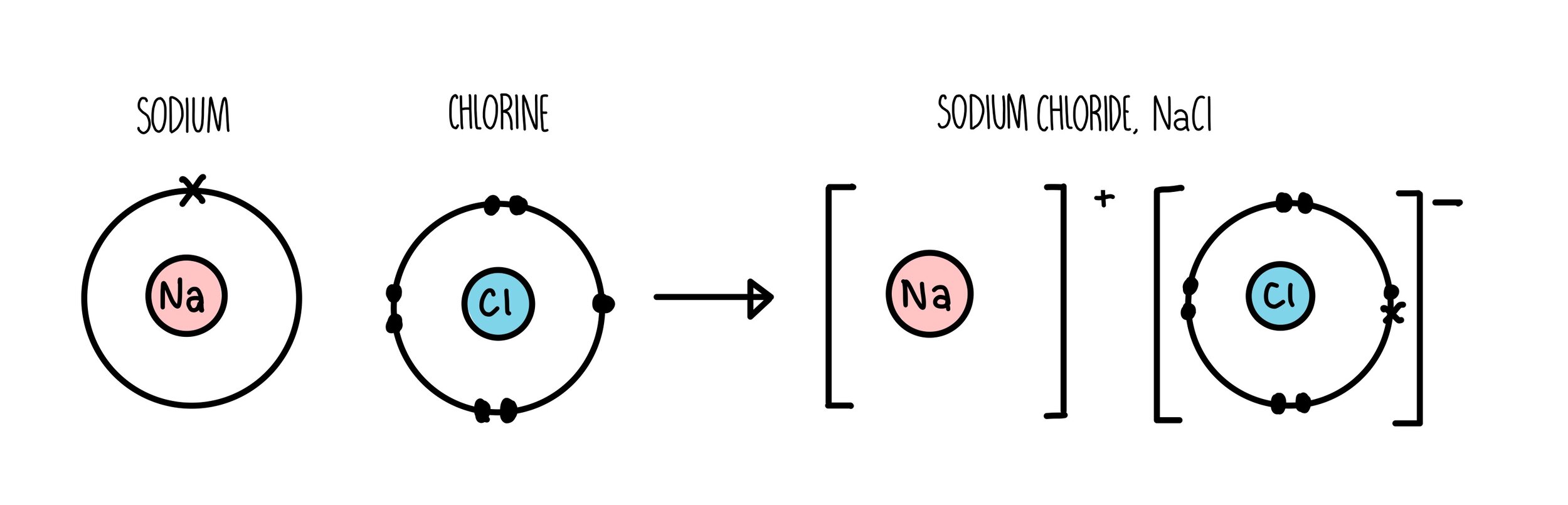
Aluminum Chloride Bond . Although aluminium chloride is covalent, when it dissolves in water, ions are produced. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Compare mgcl 2 and alcl 3. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. Aluminum and chlorine do not differ enough in. In the solid state it adopts an 'ionic lattice' structure with octahedral coordination for the alx3+ a l x 3 + ions but in the liquid and gas. Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. Aluminum chloride (alcl 3) electronegativity increases across the period; Aluminum chloride is an inorganic compound. Six water molecules bond to the aluminium to give an ion with the formula al(h 2 o) 6 3+. This gives the central aluminum atom a steric. It exists in a hydrated (alcl3.6h2o) and an anhydrous.
from www.thesciencehive.co.uk
Although aluminium chloride is covalent, when it dissolves in water, ions are produced. It exists in a hydrated (alcl3.6h2o) and an anhydrous. Six water molecules bond to the aluminium to give an ion with the formula al(h 2 o) 6 3+. In the solid state it adopts an 'ionic lattice' structure with octahedral coordination for the alx3+ a l x 3 + ions but in the liquid and gas. Aluminum and chlorine do not differ enough in. Aluminum chloride is an inorganic compound. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. Aluminum chloride (alcl 3) electronegativity increases across the period; Compare mgcl 2 and alcl 3.
Ionic Bonding — the science hive
Aluminum Chloride Bond It exists in a hydrated (alcl3.6h2o) and an anhydrous. Aluminum and chlorine do not differ enough in. Compare mgcl 2 and alcl 3. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. In the solid state it adopts an 'ionic lattice' structure with octahedral coordination for the alx3+ a l x 3 + ions but in the liquid and gas. It exists in a hydrated (alcl3.6h2o) and an anhydrous. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. Aluminum chloride is an inorganic compound. Aluminum chloride (alcl 3) electronegativity increases across the period; Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. This gives the central aluminum atom a steric. Although aluminium chloride is covalent, when it dissolves in water, ions are produced. Six water molecules bond to the aluminium to give an ion with the formula al(h 2 o) 6 3+.
From brainly.in
Ionic bond of aluminium chloride with diagram Brainly.in Aluminum Chloride Bond This gives the central aluminum atom a steric. Aluminum chloride is an inorganic compound. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. Compare mgcl 2 and alcl 3. Aluminum chloride (alcl 3) electronegativity increases across the period; The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Six water molecules bond. Aluminum Chloride Bond.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Aluminum Chloride Bond Compare mgcl 2 and alcl 3. Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. Aluminum chloride (alcl 3) electronegativity increases across the period; In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. This gives. Aluminum Chloride Bond.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Aluminum Chloride Bond Aluminum chloride (alcl 3) electronegativity increases across the period; Compare mgcl 2 and alcl 3. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Although aluminium chloride is covalent, when it dissolves in water, ions are produced. Aluminum and chlorine do not differ enough in. In this case, the aluminum atom in aluminum. Aluminum Chloride Bond.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.4.2 Representing Ionic Bonding翰林国际教育 Aluminum Chloride Bond Compare mgcl 2 and alcl 3. This gives the central aluminum atom a steric. Although aluminium chloride is covalent, when it dissolves in water, ions are produced. Six water molecules bond to the aluminium to give an ion with the formula al(h 2 o) 6 3+. It exists in a hydrated (alcl3.6h2o) and an anhydrous. Aluminum and chlorine do not. Aluminum Chloride Bond.
From schematicellsshyers.z21.web.core.windows.net
Nacl Electron Dot Diagram Aluminum Chloride Bond Aluminum chloride is an inorganic compound. Six water molecules bond to the aluminium to give an ion with the formula al(h 2 o) 6 3+. It exists in a hydrated (alcl3.6h2o) and an anhydrous. Although aluminium chloride is covalent, when it dissolves in water, ions are produced. The bond between al and cl is covalent and hence alcl 3 is. Aluminum Chloride Bond.
From present5.com
SHAPES OF MOLECULES A guide for A level Aluminum Chloride Bond It exists in a hydrated (alcl3.6h2o) and an anhydrous. This gives the central aluminum atom a steric. Aluminum chloride (alcl 3) electronegativity increases across the period; Aluminum chloride is an inorganic compound. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Mg 2+ has less polarising power than al 3+ and distorts electron. Aluminum Chloride Bond.
From www.thesciencehive.co.uk
Ionic Bonding — the science hive Aluminum Chloride Bond The bond between al and cl is covalent and hence alcl 3 is a simple molecule. This gives the central aluminum atom a steric. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. Aluminum chloride (alcl 3) electronegativity increases across the period; Alcl3 is an odorless and white/ pale yellow crystalline. Aluminum Chloride Bond.
From www.numerade.com
SOLVED Aluminum reacts with chlorine gas to form aluminum chloride via Aluminum Chloride Bond It exists in a hydrated (alcl3.6h2o) and an anhydrous. Aluminum chloride (alcl 3) electronegativity increases across the period; Six water molecules bond to the aluminium to give an ion with the formula al(h 2 o) 6 3+. Compare mgcl 2 and alcl 3. Although aluminium chloride is covalent, when it dissolves in water, ions are produced. Aluminum and chlorine do. Aluminum Chloride Bond.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Aluminum Chloride Bond Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. It exists in a hydrated (alcl3.6h2o) and an anhydrous. Aluminum and chlorine do not differ enough in. Compare mgcl 2 and alcl 3. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. In the solid state it. Aluminum Chloride Bond.
From www.youtube.com
How to Draw the Lewis Structure for AlCl3 Aluminum Chloride YouTube Aluminum Chloride Bond This gives the central aluminum atom a steric. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. Compare mgcl 2 and alcl 3. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Although aluminium chloride is covalent, when it dissolves in water, ions are. Aluminum Chloride Bond.
From www.coursehero.com
[Solved] Draw the bond between aluminum and chlorine using electron dot Aluminum Chloride Bond Compare mgcl 2 and alcl 3. Aluminum chloride is an inorganic compound. It exists in a hydrated (alcl3.6h2o) and an anhydrous. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. Although aluminium chloride is covalent, when it. Aluminum Chloride Bond.
From galvinconanstuart.blogspot.com
Electron Dot Diagram For Aluminum General Wiring Diagram Aluminum Chloride Bond Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. This gives the central aluminum atom a steric. Compare mgcl 2 and alcl 3. Six water molecules bond to the aluminium to give an ion with the formula al(h 2 o) 6 3+. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. In this. Aluminum Chloride Bond.
From www.youtube.com
Balancing Chemical Equations. Part 4 Aluminium bromide and chlorine Aluminum Chloride Bond It exists in a hydrated (alcl3.6h2o) and an anhydrous. Compare mgcl 2 and alcl 3. Aluminum chloride (alcl 3) electronegativity increases across the period; Aluminum chloride is an inorganic compound. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. In the solid state it adopts an 'ionic lattice' structure with octahedral coordination for. Aluminum Chloride Bond.
From www.mdpi.com
CMD Free FullText Comparative Study of Chloride and Fluoride Aluminum Chloride Bond Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. It exists in a hydrated (alcl3.6h2o) and an anhydrous. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. This gives the central aluminum atom a steric. Six water. Aluminum Chloride Bond.
From www.youtube.com
Ionic Bonding (Sodium + Chlorine) YouTube Aluminum Chloride Bond Aluminum chloride is an inorganic compound. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Compare mgcl 2 and alcl 3. This gives the central aluminum atom a steric. Aluminum chloride (alcl 3) electronegativity increases across the period; Six water molecules bond to the aluminium to give an ion with the formula al(h. Aluminum Chloride Bond.
From material-properties.org
Aluminium and Chlorine Comparison Properties Material Properties Aluminum Chloride Bond Compare mgcl 2 and alcl 3. Six water molecules bond to the aluminium to give an ion with the formula al(h 2 o) 6 3+. Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. Aluminum chloride (alcl 3) electronegativity increases across the period; In the solid state it adopts an 'ionic lattice' structure with octahedral. Aluminum Chloride Bond.
From www.westchem.com.au
Poly Aluminium Chloride PAC50 Able Westchem Aluminum Chloride Bond This gives the central aluminum atom a steric. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. Aluminum chloride (alcl 3) electronegativity increases across the period; Although aluminium chloride is covalent, when it dissolves in water, ions are produced. Aluminum. Aluminum Chloride Bond.
From diagrambomenslikpo.z13.web.core.windows.net
Bohr Rutherford Diagram Of Chlorine Aluminum Chloride Bond Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. This gives the central aluminum atom a steric. Although aluminium chloride is covalent, when it dissolves in water, ions are produced. Aluminum and chlorine do not differ enough in. It exists in a hydrated (alcl3.6h2o) and an anhydrous. Aluminum chloride is an inorganic compound. Six water molecules bond to. Aluminum Chloride Bond.
From www.slideserve.com
PPT A Case Study on Covalent Bonding PowerPoint Presentation, free Aluminum Chloride Bond In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. Six water molecules bond to the aluminium to give an ion with the formula al(h 2 o) 6 3+. Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. Alcl3 is an odorless and white/ pale yellow crystalline. Aluminum Chloride Bond.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.4.6 Covalent DotandCross Diagrams翰林国际教育 Aluminum Chloride Bond Although aluminium chloride is covalent, when it dissolves in water, ions are produced. Aluminum chloride is an inorganic compound. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. Six water molecules bond to the aluminium to give an ion with. Aluminum Chloride Bond.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.4.2 Representing Ionic Bonding翰林国际教育 Aluminum Chloride Bond Compare mgcl 2 and alcl 3. This gives the central aluminum atom a steric. Aluminum and chlorine do not differ enough in. In the solid state it adopts an 'ionic lattice' structure with octahedral coordination for the alx3+ a l x 3 + ions but in the liquid and gas. Although aluminium chloride is covalent, when it dissolves in water,. Aluminum Chloride Bond.
From schematiclistpact101.z22.web.core.windows.net
Diagram Of An Ionic Bond Aluminum Chloride Bond Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. Aluminum chloride is an inorganic compound. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. This gives the central aluminum atom a steric. Aluminum chloride (alcl 3) electronegativity. Aluminum Chloride Bond.
From www.youtube.com
Ionic bond formation in AlCl3(alluminium trichloride) YouTube Aluminum Chloride Bond Six water molecules bond to the aluminium to give an ion with the formula al(h 2 o) 6 3+. In the solid state it adopts an 'ionic lattice' structure with octahedral coordination for the alx3+ a l x 3 + ions but in the liquid and gas. Mg 2+ has less polarising power than al 3+ and distorts electron cloud. Aluminum Chloride Bond.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Aluminum Chloride Bond Compare mgcl 2 and alcl 3. Although aluminium chloride is covalent, when it dissolves in water, ions are produced. Aluminum and chlorine do not differ enough in. It exists in a hydrated (alcl3.6h2o) and an anhydrous. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. In this case, the aluminum atom in aluminum. Aluminum Chloride Bond.
From wiringfixprettigtp.z21.web.core.windows.net
Aluminum Electron Dot Diagram Aluminum Chloride Bond The bond between al and cl is covalent and hence alcl 3 is a simple molecule. This gives the central aluminum atom a steric. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. Aluminum chloride is an inorganic compound. In the solid state it adopts an 'ionic lattice' structure with octahedral. Aluminum Chloride Bond.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.4.6 Covalent DotandCross Diagrams翰林国际教育 Aluminum Chloride Bond Compare mgcl 2 and alcl 3. Aluminum chloride is an inorganic compound. In the solid state it adopts an 'ionic lattice' structure with octahedral coordination for the alx3+ a l x 3 + ions but in the liquid and gas. Aluminum and chlorine do not differ enough in. Although aluminium chloride is covalent, when it dissolves in water, ions are. Aluminum Chloride Bond.
From www.chemistryworld.com
Aluminium chloride Podcast Chemistry World Aluminum Chloride Bond Aluminum and chlorine do not differ enough in. Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. Aluminum chloride is an inorganic compound. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. The bond between. Aluminum Chloride Bond.
From en.wikipedia.org
Ionic bonding Wikipedia Aluminum Chloride Bond Although aluminium chloride is covalent, when it dissolves in water, ions are produced. Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. Aluminum and chlorine do not differ enough in. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. It exists in a hydrated (alcl3.6h2o) and. Aluminum Chloride Bond.
From www.science-revision.co.uk
Dative Covalent bonding Aluminum Chloride Bond Aluminum and chlorine do not differ enough in. Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. Compare mgcl 2 and alcl 3. In this case, the aluminum. Aluminum Chloride Bond.
From galvinconanstuart.blogspot.com
Electron Dot Diagram For Aluminum General Wiring Diagram Aluminum Chloride Bond The bond between al and cl is covalent and hence alcl 3 is a simple molecule. This gives the central aluminum atom a steric. Compare mgcl 2 and alcl 3. In the solid state it adopts an 'ionic lattice' structure with octahedral coordination for the alx3+ a l x 3 + ions but in the liquid and gas. Aluminum chloride. Aluminum Chloride Bond.
From aluminumchloridegoshiema.blogspot.com
Aluminum Chloride Electron Dot Diagram For Aluminum Chloride Aluminum Chloride Bond It exists in a hydrated (alcl3.6h2o) and an anhydrous. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Six water molecules bond to the aluminium to give an ion with the formula al(h 2 o) 6 3+. Although aluminium chloride is covalent,. Aluminum Chloride Bond.
From ar.inspiredpencil.com
Aluminum Chloride Structure Aluminum Chloride Bond The bond between al and cl is covalent and hence alcl 3 is a simple molecule. It exists in a hydrated (alcl3.6h2o) and an anhydrous. Aluminum and chlorine do not differ enough in. Aluminum chloride (alcl 3) electronegativity increases across the period; In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms.. Aluminum Chloride Bond.
From www.mdpi.com
CMD Free FullText Comparative Study of Chloride and Fluoride Aluminum Chloride Bond Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. It exists in a hydrated (alcl3.6h2o) and an anhydrous. Aluminum chloride is an inorganic compound. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. Aluminum chloride (alcl 3) electronegativity increases across the period; Alcl3 is an odorless. Aluminum Chloride Bond.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Aluminum Chloride Bond Mg 2+ has less polarising power than al 3+ and distorts electron cloud of. Alcl3 is an odorless and white/ pale yellow crystalline corrosive solid. Aluminum and chlorine do not differ enough in. It exists in a hydrated (alcl3.6h2o) and an anhydrous. The bond between al and cl is covalent and hence alcl 3 is a simple molecule. Six water. Aluminum Chloride Bond.
From socratic.org
What structural units make up ionic solids? Socratic Aluminum Chloride Bond Aluminum chloride (alcl 3) electronegativity increases across the period; Although aluminium chloride is covalent, when it dissolves in water, ions are produced. In this case, the aluminum atom in aluminum chloride forms three sigma bonds with the surrounding chlorine atoms. It exists in a hydrated (alcl3.6h2o) and an anhydrous. This gives the central aluminum atom a steric. Alcl3 is an. Aluminum Chloride Bond.