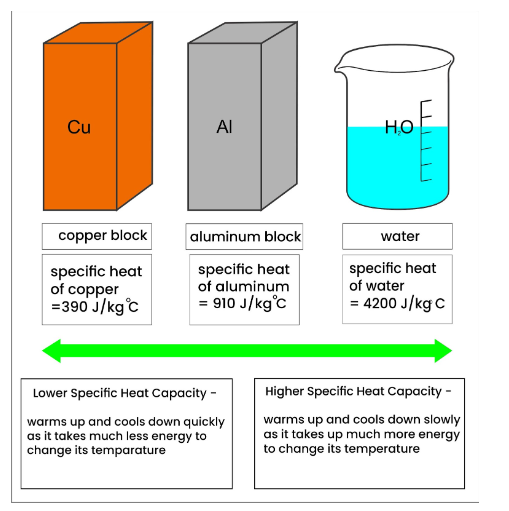
Calorimeter Has A Heat Capacity . One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calculate the heat capacity of the calorimeter in j/°c. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. 1) heat given up by warm. Calorimetry is used to measure amounts of heat transferred to or from a substance. We now introduce two concepts useful in describing heat flow and. To use calorimetric data to calculate enthalpy changes. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To do so, the heat is exchanged with a calibrated object (calorimeter). The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the.
from studymind.co.uk
We now introduce two concepts useful in describing heat flow and. Calorimetry is used to measure amounts of heat transferred to or from a substance. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. 1) heat given up by warm. To use calorimetric data to calculate enthalpy changes. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. To do so, the heat is exchanged with a calibrated object (calorimeter).
Heat Capacity Study Mind
Calorimeter Has A Heat Capacity Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: Calculate the heat capacity of the calorimeter in j/°c. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. 1) heat given up by warm. To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. We now introduce two concepts useful in describing heat flow and. To use calorimetric data to calculate enthalpy changes. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry is used to measure amounts of heat transferred to or from a substance.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Calorimeter Has A Heat Capacity Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To use calorimetric data to calculate enthalpy changes. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the.. Calorimeter Has A Heat Capacity.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimeter Has A Heat Capacity (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: We now introduce two concepts useful in describing heat flow and. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To use calorimetric data to calculate enthalpy. Calorimeter Has A Heat Capacity.
From www.youtube.com
Heat of Reaction from a Calorimeter (Example) YouTube Calorimeter Has A Heat Capacity To use calorimetric data to calculate enthalpy changes. 1) heat given up by warm. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the.. Calorimeter Has A Heat Capacity.
From 2012books.lardbucket.org
Calorimetry Calorimeter Has A Heat Capacity We now introduce two concepts useful in describing heat flow and. Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate the heat capacity of the calorimeter in j/°c. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. Calorimeter Has A Heat Capacity.
From www.education.com
Calorimetry Bomb Calorimeter Experiment Calorimeter Has A Heat Capacity Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. To use calorimetric data to calculate enthalpy changes. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of. Calorimeter Has A Heat Capacity.
From courses.lumenlearning.com
5.2 Calorimetry Chemistry Calorimeter Has A Heat Capacity Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate the heat capacity of the calorimeter in j/°c. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by. Calorimeter Has A Heat Capacity.
From www.wikihow.com
How to Calculate Heat Capacity 8 Steps (with Pictures) wikiHow Calorimeter Has A Heat Capacity Calculate the heat capacity of the calorimeter in j/°c. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. A container that prevents heat transfer in or out is called a calorimeter, and the. Calorimeter Has A Heat Capacity.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Calorimeter Has A Heat Capacity Calorimetry is used to measure amounts of heat transferred to or from a substance. To use calorimetric data to calculate enthalpy changes. Calculate the heat capacity of the calorimeter in j/°c. 1) heat given up by warm. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetery. Calorimeter Has A Heat Capacity.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimeter Has A Heat Capacity Calculate the heat capacity of the calorimeter in j/°c. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. 1) heat given up by warm. Calorimetry is used to measure amounts of heat transferred to or from a substance. (use 4.184 j. Calorimeter Has A Heat Capacity.
From www.numerade.com
SOLVED Use the References to access important values if needed for Calorimeter Has A Heat Capacity To do so, the heat is exchanged with a calibrated object (calorimeter). 1) heat given up by warm. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. A container that prevents heat transfer in or out is called a calorimeter, and the use of a. Calorimeter Has A Heat Capacity.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Calorimeter Has A Heat Capacity Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). We now introduce two concepts useful. Calorimeter Has A Heat Capacity.
From haipernews.com
How To Calculate Heat Capacity From Calorimeter Haiper Calorimeter Has A Heat Capacity One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. 1) heat given up by warm. Calculate the heat capacity of the calorimeter in j/°c. To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetery is an application of the first law of thermodynamics to. Calorimeter Has A Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Has A Heat Capacity We now introduce two concepts useful in describing heat flow and. To do so, the heat is exchanged with a calibrated object (calorimeter). 1) heat given up by warm. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: A container that prevents heat transfer in or out is called a calorimeter, and the use of. Calorimeter Has A Heat Capacity.
From www.tessshebaylo.com
Equation For Calorimetry Specific Heat Tessshebaylo Calorimeter Has A Heat Capacity Calorimetry is used to measure amounts of heat transferred to or from a substance. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: To do so, the heat is exchanged with a calibrated object (calorimeter). Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies. Calorimeter Has A Heat Capacity.
From byjus.com
What is bomb calorimeter? Calorimeter Has A Heat Capacity (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: Calorimetry is used to measure amounts of heat transferred to or from a substance. To use calorimetric data to calculate enthalpy changes. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of. Calorimeter Has A Heat Capacity.
From deon-has-edwards.blogspot.com
Heat Capacity of Calorimeter DeonhasEdwards Calorimeter Has A Heat Capacity Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. 1) heat given up by warm. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the. Calorimeter Has A Heat Capacity.
From chemistrytalk.org
Calorimetry ChemTalk Calorimeter Has A Heat Capacity (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: Calorimetry is used to measure amounts of heat transferred to or from a substance. 1) heat given up by warm. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Calculate the. Calorimeter Has A Heat Capacity.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimeter Has A Heat Capacity To do so, the heat is exchanged with a calibrated object (calorimeter). We now introduce two concepts useful in describing heat flow and. 1) heat given up by warm. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. (use. Calorimeter Has A Heat Capacity.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation ID6591088 Calorimeter Has A Heat Capacity The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. 1) heat given up by warm. A container. Calorimeter Has A Heat Capacity.
From www.expii.com
Heat Capacity of Water — Overview & Importance Expii Calorimeter Has A Heat Capacity One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. To do so, the heat is exchanged with a calibrated object (calorimeter). The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Calculate the. Calorimeter Has A Heat Capacity.
From studymind.co.uk
Heat Capacity Study Mind Calorimeter Has A Heat Capacity The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution:. Calorimeter Has A Heat Capacity.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Calorimeter Has A Heat Capacity Calculate the heat capacity of the calorimeter in j/°c. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. 1) heat given up by warm. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of. Calorimeter Has A Heat Capacity.
From www.tutorix.com
To determine specific heat capacity of given solid by method of mixtures Calorimeter Has A Heat Capacity A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. We now introduce two concepts useful in describing. Calorimeter Has A Heat Capacity.
From www.animalia-life.club
Calorimeter Diagram Calorimeter Has A Heat Capacity Calculate the heat capacity of the calorimeter in j/°c. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically. Calorimeter Has A Heat Capacity.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimeter Has A Heat Capacity Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate the heat capacity of the calorimeter in j/°c. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. Calorimetery is an application of the first law of thermodynamics to heat transfer,. Calorimeter Has A Heat Capacity.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID1875569 Calorimeter Has A Heat Capacity To use calorimetric data to calculate enthalpy changes. To do so, the heat is exchanged with a calibrated object (calorimeter). (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calculate the heat capacity. Calorimeter Has A Heat Capacity.
From studylib.net
Calorimetry Lab Specific Heat Capacity Calorimeter Has A Heat Capacity A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: Calculate the heat capacity of the calorimeter in j/°c. The temperature increase is measured and, along. Calorimeter Has A Heat Capacity.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimeter Has A Heat Capacity The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. 1) heat given up by warm. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is.. Calorimeter Has A Heat Capacity.
From www.youtube.com
050 Calorimetry YouTube Calorimeter Has A Heat Capacity Calorimetry is used to measure amounts of heat transferred to or from a substance. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: 1) heat given up by warm. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or. Calorimeter Has A Heat Capacity.
From answerhappy.com
CALORIMETRY HEAT CAPACITY OF A CALORIMETER NTRODUCTION Lab Data Calorimeter Has A Heat Capacity One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The temperature increase is measured and, along with the known heat capacity of the calorimeter, is used to calculate the energy produced by the. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution:. Calorimeter Has A Heat Capacity.
From ar.inspiredpencil.com
Common Heat Capacities Calorimeter Has A Heat Capacity A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make measurements (typically of heat or specific heat capacity) is. (use 4.184 j g¯ 1 °c¯ 1 as the specific heat of water.) solution: The temperature increase is measured and, along with the known heat capacity of the calorimeter, is. Calorimeter Has A Heat Capacity.
From users.highland.edu
Calorimetry Calorimeter Has A Heat Capacity One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. 1) heat given up by warm. To use calorimetric data to calculate enthalpy changes. We now introduce two concepts useful in describing heat flow and. Calorimetery is an application of the first law of thermodynamics to heat transfer,. Calorimeter Has A Heat Capacity.
From askfilo.com
In a constant pressure calorimeter with heat capacity of 453 Jk−1,200 mL Calorimeter Has A Heat Capacity We now introduce two concepts useful in describing heat flow and. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. A container that prevents heat transfer in or out is called a calorimeter, and the use of a calorimeter to make. Calorimeter Has A Heat Capacity.
From www.slideserve.com
PPT Specific Heat Capacity PowerPoint Presentation, free download Calorimeter Has A Heat Capacity Calorimetry is used to measure amounts of heat transferred to or from a substance. Calculate the heat capacity of the calorimeter in j/°c. To do so, the heat is exchanged with a calibrated object (calorimeter). One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. We now introduce. Calorimeter Has A Heat Capacity.
From www.mheducation.com
What is McGraw Hill Virtual Labs? McGraw Hill Higher Education Calorimeter Has A Heat Capacity Calculate the heat capacity of the calorimeter in j/°c. We now introduce two concepts useful in describing heat flow and. 1) heat given up by warm. Calorimetery is an application of the first law of thermodynamics to heat transfer, and allows us to measure the enthalpies of reaction or the heat capacities of substances. A container that prevents heat transfer. Calorimeter Has A Heat Capacity.