Electrode And Electrolyte Is . Electrode and electrolyte are two essential components in electrochemical systems. These substances constitute an important class. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrode refers to a conductor through which. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions.
from fixlibrarygedwaaldebx.z21.web.core.windows.net
When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class. Electrode and electrolyte are two essential components in electrochemical systems. Electrode refers to a conductor through which. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions.
Cathode Electrolyte Circuit Diagram
Electrode And Electrolyte Is When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode refers to a conductor through which. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. These substances constitute an important class. Electrode and electrolyte are two essential components in electrochemical systems. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood.
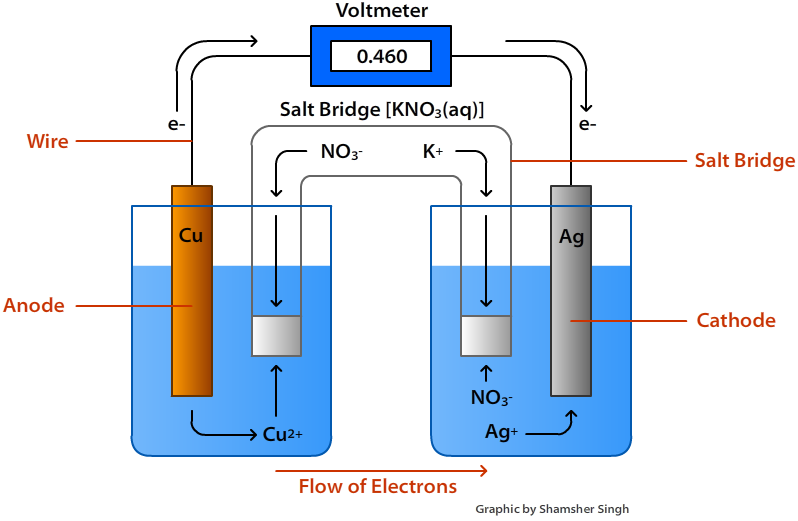
From classnotes.org.in
Electrolytic Cells Chemistry, Class 12, Electro Chemistry Electrode And Electrolyte Is Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrode and electrolyte are two essential components in electrochemical systems. Electrode refers to a conductor through which. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrodes are conductive materials. Electrode And Electrolyte Is.
From saylordotorg.github.io
Electrochemistry Electrode And Electrolyte Is Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrode and electrolyte are. Electrode And Electrolyte Is.
From courses.lumenlearning.com
11.2 Electrolytes Chemistry Electrode And Electrolyte Is Electrode refers to a conductor through which. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in. Electrode And Electrolyte Is.
From fixlibrarygedwaaldebx.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrode And Electrolyte Is When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode refers to a conductor through which. These substances constitute an important class. Electrode and electrolyte are two essential components in electrochemical systems. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in. Electrode And Electrolyte Is.
From www.youtube.com
6. Electrode and electrolyte defined (HSC chemistry) YouTube Electrode And Electrolyte Is Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class. Electrode refers to a conductor through which. Electrodes are conductive materials allowing electric current flow,. Electrode And Electrolyte Is.
From www.askdifference.com
Electrode vs. Electrolyte — What’s the Difference? Electrode And Electrolyte Is When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrode and. Electrode And Electrolyte Is.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts Electrode And Electrolyte Is Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrolytes. Electrode And Electrolyte Is.
From www.slideserve.com
PPT Biopotential Electrodes PowerPoint Presentation ID5200117 Electrode And Electrolyte Is When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode and electrolyte are two essential components in electrochemical systems. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrodes are conductive materials allowing electric current flow, whereas electrolytes. Electrode And Electrolyte Is.
From www.alamy.com
Electrolysis of water diagram. Battery, anode, cathode, cation, anion Electrode And Electrolyte Is When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode refers to a conductor through which. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolyte, substance that conducts electric current as a result of dissociation into positively and. Electrode And Electrolyte Is.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry Electrode And Electrolyte Is When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode and electrolyte are two essential components in electrochemical systems. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrode refers to a conductor through which. These substances constitute an. Electrode And Electrolyte Is.
From www.researchgate.net
The interface between electrode and electrolyte Download Scientific Electrode And Electrolyte Is Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrode and electrolyte are two essential components in electrochemical systems. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode refers to a conductor through which. Electrolyte, substance that conducts. Electrode And Electrolyte Is.
From www.worldatlas.com
What is the Difference Between a Cation and an Anion? WorldAtlas Electrode And Electrolyte Is Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode. Electrode And Electrolyte Is.
From www.researchgate.net
Schematic illustration of three types of electrolytes. Download Electrode And Electrolyte Is Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrode refers. Electrode And Electrolyte Is.
From www.batterypowertips.com
What is an electrolyte? Battery Power Tips Electrode And Electrolyte Is Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrode and electrolyte are two essential components in electrochemical systems. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrode refers to a conductor through which. When some substances are dissolved. Electrode And Electrolyte Is.
From www.mdpi.com
Energies Free FullText Electrode/Electrolyte Interphases of Sodium Electrode And Electrolyte Is When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode refers to a conductor through which. These substances constitute an important class. Electrode and electrolyte are two essential components in electrochemical systems. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such. Electrode And Electrolyte Is.
From electricalacademia.com
Voltaic Cell Working and Construction of Voltaic Cell Electrical Electrode And Electrolyte Is Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrode refers to a conductor through which. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolyte, substance that conducts electric current as a result of dissociation into positively and. Electrode And Electrolyte Is.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Electrode And Electrolyte Is Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrode and electrolyte are two essential components in electrochemical systems. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrode refers to a conductor through which. When some substances are dissolved in. Electrode And Electrolyte Is.
From www.researchgate.net
Schematic of the metal/electrolyte interface. Charge transfer takes Electrode And Electrolyte Is Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. These substances constitute an important class. Electrode refers to a conductor through which. Electrodes are conductive materials allowing electric current. Electrode And Electrolyte Is.
From pubs.acs.org
ElectrodeElectrolyte Interface in LiIon Batteries Current Electrode And Electrolyte Is These substances constitute an important class. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce. Electrode And Electrolyte Is.
From grammarbeast.com
Electrolyte vs Electrode Differences And Uses For Each One Electrode And Electrolyte Is Electrode refers to a conductor through which. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode and electrolyte are two essential components in electrochemical systems. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. These substances constitute. Electrode And Electrolyte Is.
From www.researchgate.net
Schematic of the (negative) electrodeelectrolyte interface in EDLCs Electrode And Electrolyte Is Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. These substances constitute an important class. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively. Electrode And Electrolyte Is.
From chemistry-europe.onlinelibrary.wiley.com
Understanding Electrolyte Filling of Lithium‐Ion Battery Electrodes on Electrode And Electrolyte Is These substances constitute an important class. Electrode and electrolyte are two essential components in electrochemical systems. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrodes are conductive materials. Electrode And Electrolyte Is.
From chem.libretexts.org
11.7 Electrolysis Chemistry LibreTexts Electrode And Electrolyte Is Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrode and electrolyte are two essential components in electrochemical systems. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolyte, substance that conducts electric current as a result of. Electrode And Electrolyte Is.
From mungfali.com
Reference Electrode Diagram Electrode And Electrolyte Is Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. These substances. Electrode And Electrolyte Is.
From www.researchgate.net
Electrodeelectrolyte interface model Download Scientific Diagram Electrode And Electrolyte Is Electrode and electrolyte are two essential components in electrochemical systems. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode refers to a conductor through which. Electrolytes are minerals. Electrode And Electrolyte Is.
From www.istockphoto.com
Electrode Electrolyte And Half Cell Chemistry Lesson Electrode Topic Electrode And Electrolyte Is Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrolyte, substance. Electrode And Electrolyte Is.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes Electrode And Electrolyte Is When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrode refers. Electrode And Electrolyte Is.
From www.vrogue.co
Schematic Illustration Of Electrode Electrolyte Inter vrogue.co Electrode And Electrolyte Is Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrode refers to a conductor through which. These substances constitute an important class. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrode and electrolyte are two essential components in electrochemical systems.. Electrode And Electrolyte Is.
From circuitlibscombrid.z13.web.core.windows.net
Cathode Electrolyte Circuit Diagram Electrode And Electrolyte Is Electrode and electrolyte are two essential components in electrochemical systems. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrode refers to a conductor through which. These substances constitute an important. Electrode And Electrolyte Is.
From saylordotorg.github.io
Electrochemistry Electrode And Electrolyte Is Electrode and electrolyte are two essential components in electrochemical systems. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode refers to a conductor through which. Electrodes are conductive. Electrode And Electrolyte Is.
From www.researchgate.net
Schematic of the electrodeelectrolyte interface in electrochemical Electrode And Electrolyte Is Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrode and electrolyte are two essential components in electrochemical systems. When some substances are dissolved in water, they undergo either a physical or. Electrode And Electrolyte Is.
From www.youtube.com
Electrode Skin Interface Metal Electrolyte Interface Biomedical Electrode And Electrolyte Is Electrode and electrolyte are two essential components in electrochemical systems. Electrode refers to a conductor through which. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. These substances constitute an important. Electrode And Electrolyte Is.
From www.teachoo.com
Electrolytic Cell Definition, Components, Examples Teachoo Electrode And Electrolyte Is When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. These substances. Electrode And Electrolyte Is.
From chem.libretexts.org
5.6 Day 41 Electrolysis; Commercial Batteries Chemistry LibreTexts Electrode And Electrolyte Is Electrolyte, substance that conducts electric current as a result of dissociation into positively and negatively charged particles called ions. When some substances are dissolved in water, they undergo either a physical or a chemical change that yields ions in solution. Electrode refers to a conductor through which. Electrolytes are minerals that carry an electric charge when they are dissolved in. Electrode And Electrolyte Is.
From www.researchgate.net
ElectrodeElectrolyte Interface Download Scientific Diagram Electrode And Electrolyte Is Electrode and electrolyte are two essential components in electrochemical systems. Electrolytes are minerals that carry an electric charge when they are dissolved in a liquid, such as blood. Electrode refers to a conductor through which. Electrodes are conductive materials allowing electric current flow, whereas electrolytes are substances that produce ions in solution, enabling conductivity. Electrolyte, substance that conducts electric current. Electrode And Electrolyte Is.