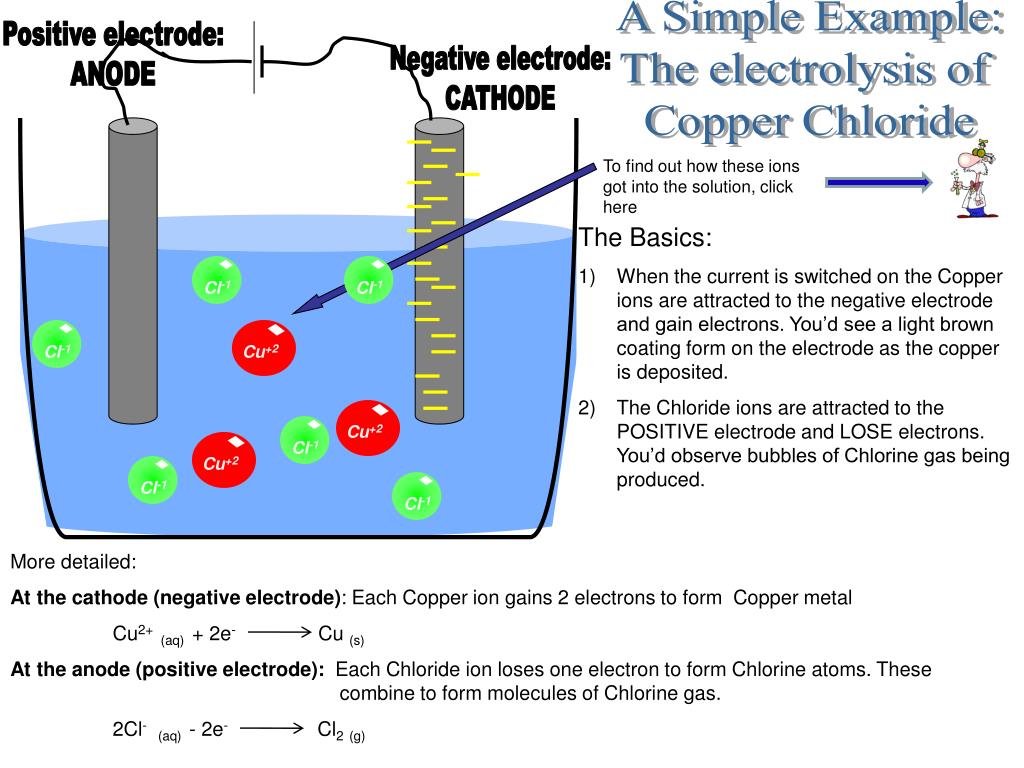
What Is The Best Electrode For Electrolysis . Ionic compounds conduct electricity when molten or in solution. magnesium sulfate is not the best choice when carrying out an electrolysis. In electroplating, the object to be plated is made the cathode. Ionic compounds conduct electricity when molten or in. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. The role of water in the electrolysis of aqueous solutions of electrolytes. reactive metals are extracted from their ores using electrolysis. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. reactive metals are extracted from their ores using electrolysis. The situation is more complicated when you electrolyse a solution.
from www.slideserve.com
The situation is more complicated when you electrolyse a solution. Ionic compounds conduct electricity when molten or in solution. magnesium sulfate is not the best choice when carrying out an electrolysis. The role of water in the electrolysis of aqueous solutions of electrolytes. Ionic compounds conduct electricity when molten or in. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. In electroplating, the object to be plated is made the cathode. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. reactive metals are extracted from their ores using electrolysis.
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367
What Is The Best Electrode For Electrolysis reactive metals are extracted from their ores using electrolysis. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. reactive metals are extracted from their ores using electrolysis. magnesium sulfate is not the best choice when carrying out an electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. The situation is more complicated when you electrolyse a solution. In electroplating, the object to be plated is made the cathode. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. Ionic compounds conduct electricity when molten or in solution. Ionic compounds conduct electricity when molten or in. The role of water in the electrolysis of aqueous solutions of electrolytes. reactive metals are extracted from their ores using electrolysis.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte What Is The Best Electrode For Electrolysis The situation is more complicated when you electrolyse a solution. reactive metals are extracted from their ores using electrolysis. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in solution. In electroplating, the object to be plated is made the cathode. The role of water in the electrolysis of aqueous solutions. What Is The Best Electrode For Electrolysis.
From phys.org
A powerful catalyst for electrolysis of water that could help harness What Is The Best Electrode For Electrolysis Ionic compounds conduct electricity when molten or in. In electroplating, the object to be plated is made the cathode. reactive metals are extracted from their ores using electrolysis. magnesium sulfate is not the best choice when carrying out an electrolysis. Ionic compounds conduct electricity when molten or in solution. in any electrochemical cell (electrolytic or galvanic) the. What Is The Best Electrode For Electrolysis.
From www.youtube.com
what is the meaning of electrolysis chemistry electrodes What Is The Best Electrode For Electrolysis magnesium sulfate is not the best choice when carrying out an electrolysis. The role of water in the electrolysis of aqueous solutions of electrolytes. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. reactive metals are extracted from their ores using electrolysis. . What Is The Best Electrode For Electrolysis.
From dxoargcof.blob.core.windows.net
Electrodes Electrolysis Experiment at Cindy Hopson blog What Is The Best Electrode For Electrolysis magnesium sulfate is not the best choice when carrying out an electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. The situation is. What Is The Best Electrode For Electrolysis.
From philschatz.com
Electrolysis · Chemistry What Is The Best Electrode For Electrolysis Ionic compounds conduct electricity when molten or in solution. magnesium sulfate is not the best choice when carrying out an electrolysis. The role of water in the electrolysis of aqueous solutions of electrolytes. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. In electroplating, the object to be plated is made. What Is The Best Electrode For Electrolysis.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts What Is The Best Electrode For Electrolysis in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. Ionic compounds conduct electricity when molten or in solution. The situation is more complicated when you electrolyse a solution. The role of water. What Is The Best Electrode For Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes What Is The Best Electrode For Electrolysis Ionic compounds conduct electricity when molten or in. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. magnesium sulfate is not the best choice when carrying out an electrolysis. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. electrolysis. What Is The Best Electrode For Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk What Is The Best Electrode For Electrolysis The role of water in the electrolysis of aqueous solutions of electrolytes. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. The situation is more complicated when you. What Is The Best Electrode For Electrolysis.
From courses.lumenlearning.com
Electrolysis Boundless Chemistry What Is The Best Electrode For Electrolysis In electroplating, the object to be plated is made the cathode. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. Ionic compounds conduct electricity when molten or in. The situation is more complicated when you electrolyse a solution. reactive metals are extracted from their ores using electrolysis. the electrode where. What Is The Best Electrode For Electrolysis.
From www.researchgate.net
Schematic illustration of the 3electrode electrolytic cell and the What Is The Best Electrode For Electrolysis reactive metals are extracted from their ores using electrolysis. The role of water in the electrolysis of aqueous solutions of electrolytes. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or in. the electrode. What Is The Best Electrode For Electrolysis.
From brilliant.org
Electrolytic Cells and Electrolysis Brilliant Math & Science Wiki What Is The Best Electrode For Electrolysis the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. Ionic compounds conduct electricity when molten or in. magnesium sulfate is not the best choice when carrying out an electrolysis. In electroplating, the object to be plated is made the cathode. electrolysis can occur in electrolytic cells by. What Is The Best Electrode For Electrolysis.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID9353258 What Is The Best Electrode For Electrolysis the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. reactive metals are extracted from their ores using electrolysis. reactive metals are extracted from their ores using electrolysis. In electroplating, the object to be plated is made the cathode. The situation is more complicated when you electrolyse a. What Is The Best Electrode For Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace What Is The Best Electrode For Electrolysis The situation is more complicated when you electrolyse a solution. Ionic compounds conduct electricity when molten or in solution. reactive metals are extracted from their ores using electrolysis. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. reactive metals are extracted from their ores using electrolysis. . What Is The Best Electrode For Electrolysis.
From www.snexplores.org
Explainer What is an electrode? What Is The Best Electrode For Electrolysis reactive metals are extracted from their ores using electrolysis. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. In electroplating, the object to be plated is made the cathode. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow. What Is The Best Electrode For Electrolysis.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 What Is The Best Electrode For Electrolysis in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. reactive metals are extracted from their ores using electrolysis. The role of water in the electrolysis of aqueous solutions of electrolytes. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to. What Is The Best Electrode For Electrolysis.
From www.slideserve.com
PPT 17.78 Electrolysis & Applications PowerPoint Presentation ID What Is The Best Electrode For Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. reactive metals are extracted from their ores using electrolysis. The situation is more complicated when you electrolyse a solution. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs. What Is The Best Electrode For Electrolysis.
From www.researchgate.net
Schematic of the membrane electrode assembly (MEA) and catalyst What Is The Best Electrode For Electrolysis Ionic compounds conduct electricity when molten or in solution. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. reactive metals are extracted from their ores using electrolysis. The role of water in the electrolysis of aqueous solutions of electrolytes. magnesium sulfate is not. What Is The Best Electrode For Electrolysis.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download What Is The Best Electrode For Electrolysis reactive metals are extracted from their ores using electrolysis. The situation is more complicated when you electrolyse a solution. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. Ionic compounds conduct electricity when molten or in solution. reactive metals are extracted from their. What Is The Best Electrode For Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 What Is The Best Electrode For Electrolysis The situation is more complicated when you electrolyse a solution. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons. What Is The Best Electrode For Electrolysis.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic What Is The Best Electrode For Electrolysis In electroplating, the object to be plated is made the cathode. The situation is more complicated when you electrolyse a solution. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply,. What Is The Best Electrode For Electrolysis.
From byjus.com
Water Electrolysis Principle of Water Electrolysis, Important Factors What Is The Best Electrode For Electrolysis reactive metals are extracted from their ores using electrolysis. The role of water in the electrolysis of aqueous solutions of electrolytes. magnesium sulfate is not the best choice when carrying out an electrolysis. Ionic compounds conduct electricity when molten or in. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the.. What Is The Best Electrode For Electrolysis.
From courses.lumenlearning.com
Electrolysis Chemistry for Majors What Is The Best Electrode For Electrolysis Ionic compounds conduct electricity when molten or in. The situation is more complicated when you electrolyse a solution. reactive metals are extracted from their ores using electrolysis. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. the electrode where oxidation occurs is called the anode and the electrode where reduction. What Is The Best Electrode For Electrolysis.
From www.youtube.com
Three Electrode Electrolysis YouTube What Is The Best Electrode For Electrolysis the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. The situation is more complicated when you electrolyse a solution. The role of water in the. What Is The Best Electrode For Electrolysis.
From www.alamy.com
Diagram of the electrolysis of copper (II) chromate (VI) showing the What Is The Best Electrode For Electrolysis In electroplating, the object to be plated is made the cathode. reactive metals are extracted from their ores using electrolysis. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. Ionic compounds. What Is The Best Electrode For Electrolysis.
From www.alamy.com
Electrolysis process vector illustration. Simple electrolysis process What Is The Best Electrode For Electrolysis magnesium sulfate is not the best choice when carrying out an electrolysis. In electroplating, the object to be plated is made the cathode. The situation is more complicated when you electrolyse a solution. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. the electrode where oxidation occurs is called the. What Is The Best Electrode For Electrolysis.
From exowuasqn.blob.core.windows.net
Stainless Steel Electrodes For Electrolysis at Loretta Bryan blog What Is The Best Electrode For Electrolysis the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. In electroplating, the object to be plated is made the cathode. The role of water in the electrolysis of aqueous solutions of electrolytes. reactive metals are extracted from their ores using electrolysis. Ionic compounds conduct electricity when molten or. What Is The Best Electrode For Electrolysis.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube What Is The Best Electrode For Electrolysis electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. The role of water in the electrolysis of aqueous solutions of electrolytes. In electroplating, the object. What Is The Best Electrode For Electrolysis.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis What Is The Best Electrode For Electrolysis The situation is more complicated when you electrolyse a solution. The role of water in the electrolysis of aqueous solutions of electrolytes. reactive metals are extracted from their ores using electrolysis. reactive metals are extracted from their ores using electrolysis. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called. What Is The Best Electrode For Electrolysis.
From general.chemistrysteps.com
Electrolysis Chemistry Steps What Is The Best Electrode For Electrolysis The situation is more complicated when you electrolyse a solution. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. Ionic compounds conduct electricity when molten or in. magnesium sulfate is not the best choice. What Is The Best Electrode For Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID6499367 What Is The Best Electrode For Electrolysis the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. reactive metals are extracted from their ores using electrolysis. The situation is more complicated when you electrolyse a solution. In electroplating, the object to be plated is made the cathode. in any electrochemical cell (electrolytic or galvanic) the. What Is The Best Electrode For Electrolysis.
From study.com
Electrolysis of Aqueous Solutions Lesson What Is The Best Electrode For Electrolysis reactive metals are extracted from their ores using electrolysis. The situation is more complicated when you electrolyse a solution. The role of water in the electrolysis of aqueous solutions of electrolytes. Ionic compounds conduct electricity when molten or in solution. magnesium sulfate is not the best choice when carrying out an electrolysis. Ionic compounds conduct electricity when molten. What Is The Best Electrode For Electrolysis.
From spmscience.blog.onlinetuition.com.my
Electrolysis SPM Science What Is The Best Electrode For Electrolysis In electroplating, the object to be plated is made the cathode. Ionic compounds conduct electricity when molten or in. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. The situation is more complicated when you electrolyse a solution. The role of water in the electrolysis. What Is The Best Electrode For Electrolysis.
From www.researchgate.net
10 Schematic diagram of three electrode electrolytic system for What Is The Best Electrode For Electrolysis In electroplating, the object to be plated is made the cathode. reactive metals are extracted from their ores using electrolysis. the electrode where oxidation occurs is called the anode and the electrode where reduction occurs is called the cathode. magnesium sulfate is not the best choice when carrying out an electrolysis. in any electrochemical cell (electrolytic. What Is The Best Electrode For Electrolysis.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis What Is The Best Electrode For Electrolysis reactive metals are extracted from their ores using electrolysis. reactive metals are extracted from their ores using electrolysis. electrolysis can occur in electrolytic cells by introducing a power supply, which supplies the energy to force the electrons to flow in the. In electroplating, the object to be plated is made the cathode. The role of water in. What Is The Best Electrode For Electrolysis.
From diagramlibrarypern.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis What Is The Best Electrode For Electrolysis Ionic compounds conduct electricity when molten or in. reactive metals are extracted from their ores using electrolysis. magnesium sulfate is not the best choice when carrying out an electrolysis. in any electrochemical cell (electrolytic or galvanic) the electrode at which reduction occurs is called the. reactive metals are extracted from their ores using electrolysis. the. What Is The Best Electrode For Electrolysis.