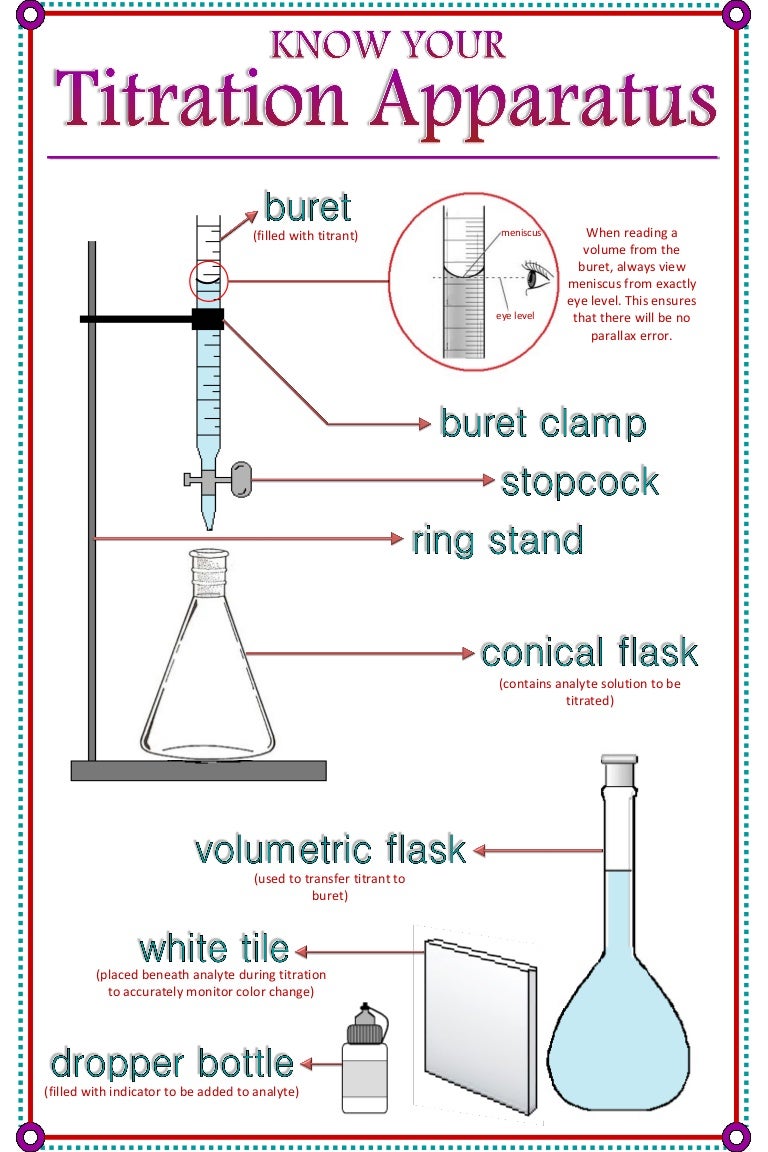
Titration In Chemistry Table . Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. What are titrant and analyte. What is the equivalence point of a titration. There are two basic types of acid base titrations, indicator and potentiometric. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. However, the most common types of titration in. In an indicator based titration you add another chemical that changes. The basic process involves adding a. There are many types of titration when considering goals and procedures. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It is based on the neutralization reaction, where. Learn the titration graph and titration equation.
from letitsnowglobe.co.uk
The basic process involves adding a. In an indicator based titration you add another chemical that changes. However, the most common types of titration in. It is based on the neutralization reaction, where. There are two basic types of acid base titrations, indicator and potentiometric. Learn the titration graph and titration equation. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. What is the equivalence point of a titration. What are titrant and analyte. There are many types of titration when considering goals and procedures.
Titration procedure pdf
Titration In Chemistry Table A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. Learn the titration graph and titration equation. What is the equivalence point of a titration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In an indicator based titration you add another chemical that changes. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. What are titrant and analyte. However, the most common types of titration in. There are many types of titration when considering goals and procedures. There are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where.
From www.youtube.com
TYPES OF TITRATION IN CHEMISTRY ACID BASE TITRATION REDOX TITRATION Titration In Chemistry Table Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Learn the titration graph and titration equation. There are two basic types of. Titration In Chemistry Table.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration In Chemistry Table There are many types of titration when considering goals and procedures. However, the most common types of titration in. Learn the titration graph and titration equation. There are two basic types of acid base titrations, indicator and potentiometric. What is the equivalence point of a titration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be. Titration In Chemistry Table.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Titration In Chemistry Table As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. However, the most common types of titration in. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. What is the equivalence point of a titration. Titration. Titration In Chemistry Table.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Titration In Chemistry Table It is based on the neutralization reaction, where. Learn the titration graph and titration equation. The basic process involves adding a. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. What is the equivalence point of a titration. However, the most common types of titration. Titration In Chemistry Table.
From www.studocu.com
Titration Lab Report Acid Base Titration Lab Report Table 3 NaOH Titration In Chemistry Table What are titrant and analyte. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Learn the titration graph and titration equation. What is the equivalence point of a titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration In Chemistry Table.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration In Chemistry Table What is the equivalence point of a titration. Learn the titration graph and titration equation. It is based on the neutralization reaction, where. There are many types of titration when considering goals and procedures. There are two basic types of acid base titrations, indicator and potentiometric. What are titrant and analyte. Titration is the slow addition of one solution of. Titration In Chemistry Table.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry Titration In Chemistry Table As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In an indicator based titration you add another chemical that changes. Learn the titration graph and titration equation. What are titrant and analyte. What is the equivalence point of a titration. However, the most common types of. Titration In Chemistry Table.
From www.compoundchem.com
Chemistry Techniques Titration Compound Interest Titration In Chemistry Table There are many types of titration when considering goals and procedures. What are titrant and analyte. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Titration. Titration In Chemistry Table.
From www.chegg.com
Solved Results Titration Tables Table 5. Titration of Titration In Chemistry Table There are two basic types of acid base titrations, indicator and potentiometric. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. There are many types of titration when considering goals and procedures. The basic process involves adding a. A titration is a laboratory technique used. Titration In Chemistry Table.
From chem.libretexts.org
Chapter 16.5 AcidBase Titrations Chemistry LibreTexts Titration In Chemistry Table What is the equivalence point of a titration. There are two basic types of acid base titrations, indicator and potentiometric. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. However, the most common types of titration in. In an indicator based titration you add another. Titration In Chemistry Table.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Titration In Chemistry Table The basic process involves adding a. There are many types of titration when considering goals and procedures. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another chemical that changes. What are titrant and analyte. Learn the titration graph and titration equation. Titration is the slow addition of one solution. Titration In Chemistry Table.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Titration In Chemistry Table There are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Titration is the slow addition of one solution of a known concentration (called a titrant) to a. Titration In Chemistry Table.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube Titration In Chemistry Table It is based on the neutralization reaction, where. However, the most common types of titration in. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. There are many types of titration when considering goals and procedures. The basic process involves adding a. There are two. Titration In Chemistry Table.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration In Chemistry Table There are two basic types of acid base titrations, indicator and potentiometric. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Learn the titration graph and titration equation. What is the equivalence point of a titration. It is based on the neutralization reaction, where. A. Titration In Chemistry Table.
From theedge.com.hk
Chemistry How To Titration The Edge Titration In Chemistry Table There are two basic types of acid base titrations, indicator and potentiometric. What is the equivalence point of a titration. The basic process involves adding a. What are titrant and analyte. Learn the titration graph and titration equation. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. However, the. Titration In Chemistry Table.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration In Chemistry Table However, the most common types of titration in. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. There are many. Titration In Chemistry Table.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration In Chemistry Table Learn the titration graph and titration equation. What is the equivalence point of a titration. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. There are many types of titration when considering goals and procedures. In an indicator based titration you add another chemical that changes.. Titration In Chemistry Table.
From www.chegg.com
Solved Section Titration Lab Report Sheet Table 1 Acid Base Titration In Chemistry Table Learn the titration graph and titration equation. However, the most common types of titration in. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic. Titration In Chemistry Table.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration In Chemistry Table What are titrant and analyte. There are two basic types of acid base titrations, indicator and potentiometric. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Titration In Chemistry Table.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube Titration In Chemistry Table Learn the titration graph and titration equation. There are two basic types of acid base titrations, indicator and potentiometric. There are many types of titration when considering goals and procedures. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. What are titrant and analyte. However, the. Titration In Chemistry Table.
From www.pinterest.com
UCDavis Titration Procedure/Labels Bildung Chemistry education Titration In Chemistry Table A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. In an indicator based titration you add another chemical that changes. There are two basic types of. Titration In Chemistry Table.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration In Chemistry Table Learn the titration graph and titration equation. There are two basic types of acid base titrations, indicator and potentiometric. There are many types of titration when considering goals and procedures. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. However, the most common types of titration. Titration In Chemistry Table.
From www.youtube.com
Titration Calculations National 5 Chemistry Lesson 5 YouTube Titration In Chemistry Table What are titrant and analyte. Learn the titration graph and titration equation. There are two basic types of acid base titrations, indicator and potentiometric. It is based on the neutralization reaction, where. There are many types of titration when considering goals and procedures. The basic process involves adding a. As seen in the chapter on the stoichiometry of chemical reactions,. Titration In Chemistry Table.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration In Chemistry Table What are titrant and analyte. There are two basic types of acid base titrations, indicator and potentiometric. The basic process involves adding a. What is the equivalence point of a titration. It is based on the neutralization reaction, where. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration. Titration In Chemistry Table.
From mungfali.com
Acid Base Titration Calculation Titration In Chemistry Table However, the most common types of titration in. There are many types of titration when considering goals and procedures. In an indicator based titration you add another chemical that changes. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Titration is the slow addition of one. Titration In Chemistry Table.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration In Chemistry Table There are many types of titration when considering goals and procedures. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. There are two basic types of acid base titrations, indicator and potentiometric. What are titrant and analyte. Titration is the slow addition of one solution of. Titration In Chemistry Table.
From letitsnowglobe.co.uk
Titration procedure pdf Titration In Chemistry Table In an indicator based titration you add another chemical that changes. The basic process involves adding a. It is based on the neutralization reaction, where. Learn the titration graph and titration equation. However, the most common types of titration in. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of. Titration In Chemistry Table.
From www.thestudentroom.co.uk
AS Chemistry Titration The Student Room Titration In Chemistry Table A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. It is based on the neutralization reaction, where. However, the most common types of titration in. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Learn. Titration In Chemistry Table.
From www.savemyexams.com
pH Titration Curves OCR A Level Chemistry Revision Notes 2017 Titration In Chemistry Table What are titrant and analyte. It is based on the neutralization reaction, where. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. What is the equivalence. Titration In Chemistry Table.
From www.vrogue.co
Titration Calculations Science Chemistry Chemical Rea vrogue.co Titration In Chemistry Table In an indicator based titration you add another chemical that changes. What are titrant and analyte. However, the most common types of titration in. The basic process involves adding a. There are many types of titration when considering goals and procedures. It is based on the neutralization reaction, where. A titration is a laboratory technique used to precisely measure molar. Titration In Chemistry Table.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Titration In Chemistry Table What are titrant and analyte. What is the equivalence point of a titration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. In an indicator based titration you add another chemical that changes. It is based on the neutralization reaction, where. However, the most common. Titration In Chemistry Table.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Titration In Chemistry Table There are two basic types of acid base titrations, indicator and potentiometric. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In an indicator based titration you add another chemical that changes. However, the most common types of titration in. What is the equivalence point of a titration. There. Titration In Chemistry Table.
From studylib.net
CHEMISTRY I LAB INTRO TO TITRATION Titration In Chemistry Table As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. What are titrant and analyte. Learn the titration graph and titration equation. The basic process involves adding a. What is the equivalence point of a titration. There are many types of titration when considering goals and procedures.. Titration In Chemistry Table.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations* — the science sauce Titration In Chemistry Table The basic process involves adding a. However, the most common types of titration in. It is based on the neutralization reaction, where. In an indicator based titration you add another chemical that changes. There are many types of titration when considering goals and procedures. Titration is the slow addition of one solution of a known concentration (called a titrant) to. Titration In Chemistry Table.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Titration In Chemistry Table A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. There are many types of titration when considering goals and procedures. What are titrant and analyte. It is based on the neutralization reaction, where. However, the most common types of titration in. In an indicator based titration you add another. Titration In Chemistry Table.