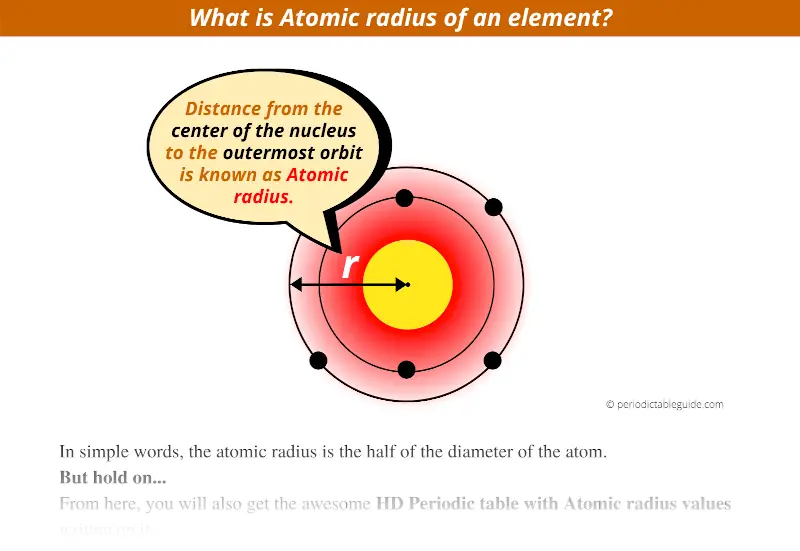
What Is Atomic Radius Affected By . This is caused by the increase in the number of protons and electrons across a period. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. In simpler terms, it can be defined as something similar to the. The size of the atoms of. Here are the definitions of atomic and ionic radius, the difference between. The bond length of a diatomic. The atomic radius of atoms generally. The radius of an atom is the distance from the center of its nucleus to its outermost electrons. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Atomic radius decreases from left to right within a period. Bond length from atomic radii. Atomic radius and ionic radius are two of the most common atom size measurements. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal:
from periodictableguide.com
Bond length from atomic radii. For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal: Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. The atomic radius of atoms generally. Atomic radius and ionic radius are two of the most common atom size measurements. The size of the atoms of. In simpler terms, it can be defined as something similar to the. This is caused by the increase in the number of protons and electrons across a period. Here are the definitions of atomic and ionic radius, the difference between. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron.
Get the Periodic table with Atomic radius values (Img+Chart)
What Is Atomic Radius Affected By Here are the definitions of atomic and ionic radius, the difference between. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Bond length from atomic radii. Here are the definitions of atomic and ionic radius, the difference between. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. The radius of an atom is the distance from the center of its nucleus to its outermost electrons. One proton has a greater effect than one. The bond length of a diatomic. This is caused by the increase in the number of protons and electrons across a period. Atomic radius and ionic radius are two of the most common atom size measurements. For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal: The atomic radius of atoms generally. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. We define the atomic radius of a chemical element. Atomic radius decreases from left to right within a period. The size of the atoms of.
From worksheetsufertatstl.z21.web.core.windows.net
Atomic Radius Trend Examples What Is Atomic Radius Affected By Here are the definitions of atomic and ionic radius, the difference between. The bond length of a diatomic. The radius of an atom is the distance from the center of its nucleus to its outermost electrons. Atomic radius decreases from left to right within a period. This is caused by the increase in the number of protons and electrons across. What Is Atomic Radius Affected By.
From study.com
What is Atomic Radius? Atomic Radius Examples & Periodic Trend What Is Atomic Radius Affected By The atomic radius of atoms generally. In simpler terms, it can be defined as something similar to the. One proton has a greater effect than one. This is caused by the increase in the number of protons and electrons across a period. Bond length from atomic radii. The bond length of a diatomic. Atomic radius or atomic radii is the. What Is Atomic Radius Affected By.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table What Is Atomic Radius Affected By Bond length from atomic radii. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. This is caused by the increase in the number of protons and electrons across a period.. What Is Atomic Radius Affected By.
From lavelle.chem.ucla.edu
Chapter 2 HW 93 CHEMISTRY COMMUNITY What Is Atomic Radius Affected By The size of the atoms of. For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal: Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. The radius of an atom is the distance from the center of its. What Is Atomic Radius Affected By.
From slideplayer.com
Periodic Trends. ppt download What Is Atomic Radius Affected By One proton has a greater effect than one. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. In simpler terms, it can. What Is Atomic Radius Affected By.
From periodictable.me
list of atomic radius What Is Atomic Radius Affected By The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. One proton has a greater effect than one. Atomic radius and ionic radius are two of the most common atom size measurements. Atomic radius decreases from left to right within a period. Atomic. What Is Atomic Radius Affected By.
From ar.inspiredpencil.com
Measuring Atomic Radius What Is Atomic Radius Affected By Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. The bond length of a diatomic. We define the atomic radius of a chemical element. Bond length from atomic radii. One proton has a greater effect than one. Atomic radius decreases from left to right within a period.. What Is Atomic Radius Affected By.
From sciencetrends.com
Atomic Radius Trend Science Trends What Is Atomic Radius Affected By The size of the atoms of. Bond length from atomic radii. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. The atomic radius of atoms generally. We define the atomic radius of a chemical element. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the. What Is Atomic Radius Affected By.
From freeloadshat.weebly.com
freeloadshat Blog What Is Atomic Radius Affected By The radius of an atom is the distance from the center of its nucleus to its outermost electrons. Here are the definitions of atomic and ionic radius, the difference between. The atomic radius of atoms generally. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. We define the atomic radius of a. What Is Atomic Radius Affected By.
From facts.net
12 Intriguing Facts About Effective Atomic Radius What Is Atomic Radius Affected By For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal: The atomic radius of atoms generally. The size of the atoms of. The radius of an atom is the distance from the center of its nucleus to its outermost electrons. Atomic radius is determined as half the distance between. What Is Atomic Radius Affected By.
From www.sciencealert.com
This Nuclear Bomb Map Shows What Would Happen if One Exploded Near You What Is Atomic Radius Affected By One proton has a greater effect than one. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. This is caused by the increase in the number of protons and electrons across a period. Bond length from atomic radii. Atomic radius is generally stated as being the total distance from an atom’s nucleus. What Is Atomic Radius Affected By.
From askfilo.com
Fig. 3.4 (b) Variation of atomic radius with atomic number for alkali met.. What Is Atomic Radius Affected By Atomic radius decreases from left to right within a period. Atomic radius and ionic radius are two of the most common atom size measurements. One proton has a greater effect than one. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. This is caused by the increase in the number of protons. What Is Atomic Radius Affected By.
From www.youtube.com
What is Atomic Radius. YouTube What Is Atomic Radius Affected By We define the atomic radius of a chemical element. For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal: Here are the definitions of atomic and ionic radius, the difference between. The radius of an atom is the distance from the center of its nucleus to its outermost electrons.. What Is Atomic Radius Affected By.
From neetlab.com
Atomic Radius NEETLab What Is Atomic Radius Affected By The atomic radius of atoms generally. This is caused by the increase in the number of protons and electrons across a period. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. For metals, the atomic radius is defined as half the distance. What Is Atomic Radius Affected By.
From mcatmastery.net
Periodic Table on the MCAT MCAT Mastery What Is Atomic Radius Affected By For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal: The atomic radius of atoms generally. The size of the atoms of. Atomic radius decreases from left to right within a period. In simpler terms, it can be defined as something similar to the. The bond length of a. What Is Atomic Radius Affected By.
From www.pearson.com
Given the atomic radius of the elementsDetermine the atomic radiu What Is Atomic Radius Affected By The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. One proton has a greater effect than one. The bond length of a diatomic. In simpler terms, it can be defined as something similar to the. We define the atomic radius of a. What Is Atomic Radius Affected By.
From www.trentu.ca
PreChemistry What Is Atomic Radius Affected By The bond length of a diatomic. For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal: The size of the atoms of. We define the atomic radius of a chemical element. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost. What Is Atomic Radius Affected By.
From stock.adobe.com
types of atomic radius of hydrogen chemical element. Ionic radius What Is Atomic Radius Affected By Bond length from atomic radii. The radius of an atom is the distance from the center of its nucleus to its outermost electrons. We define the atomic radius of a chemical element. In simpler terms, it can be defined as something similar to the. This is caused by the increase in the number of protons and electrons across a period.. What Is Atomic Radius Affected By.
From ezchem.com
Atomic Radius Trend EZchem What Is Atomic Radius Affected By The size of the atoms of. Here are the definitions of atomic and ionic radius, the difference between. The bond length of a diatomic. We define the atomic radius of a chemical element. The radius of an atom is the distance from the center of its nucleus to its outermost electrons. One proton has a greater effect than one. This. What Is Atomic Radius Affected By.
From 88guru.com
Atomic RadiusAn Overview 88Guru What Is Atomic Radius Affected By In simpler terms, it can be defined as something similar to the. One proton has a greater effect than one. The radius of an atom is the distance from the center of its nucleus to its outermost electrons. Atomic radius and ionic radius are two of the most common atom size measurements. Atomic radius is determined as half the distance. What Is Atomic Radius Affected By.
From chamotgallery.com
Understanding Atomic Radius Trends The 2 Key Principles (2023) What Is Atomic Radius Affected By One proton has a greater effect than one. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. The radius of an atom is the distance from the center of its nucleus to its outermost electrons. This is caused by the increase in the number of protons and. What Is Atomic Radius Affected By.
From pediabay.com
Atomic Radius of Elements (With Periodic table Chart) Pediabay What Is Atomic Radius Affected By Here are the definitions of atomic and ionic radius, the difference between. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal: Atomic radius and ionic radius are two of the most. What Is Atomic Radius Affected By.
From www.youtube.com
What causes atomic radius to increase? YouTube What Is Atomic Radius Affected By The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the. This is caused by the increase in the number of protons and electrons across a period. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost. What Is Atomic Radius Affected By.
From www.breakingatom.com
Atomic Radius of Elements What Is Atomic Radius Affected By Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. One proton has a greater effect than one. We define the atomic radius of a chemical element. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic radius or atomic radii is. What Is Atomic Radius Affected By.
From ezchem.com
Atomic Radius Trend EZchem What Is Atomic Radius Affected By Atomic radius and ionic radius are two of the most common atom size measurements. This is caused by the increase in the number of protons and electrons across a period. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. The bond length of a diatomic. Bond length from atomic radii. Here are. What Is Atomic Radius Affected By.
From slideplayer.com
Trends & the Periodic Table ppt download What Is Atomic Radius Affected By Here are the definitions of atomic and ionic radius, the difference between. The atomic radius of atoms generally. The bond length of a diatomic. The radius of an atom is the distance from the center of its nucleus to its outermost electrons. This is caused by the increase in the number of protons and electrons across a period. One proton. What Is Atomic Radius Affected By.
From slideplayer.com
WARM UP Write configurations for the following elements, using noble What Is Atomic Radius Affected By One proton has a greater effect than one. Atomic radius decreases from left to right within a period. Atomic radius and ionic radius are two of the most common atom size measurements. Bond length from atomic radii. The radius of an atom is the distance from the center of its nucleus to its outermost electrons. In simpler terms, it can. What Is Atomic Radius Affected By.
From flexbooks.ck12.org
CK12Foundation What Is Atomic Radius Affected By The atomic radius of atoms generally. The bond length of a diatomic. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of electron. Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. Atomic radius is determined as half. What Is Atomic Radius Affected By.
From surfguppy.com
What is Electronegativity? What Is Atomic Radius Affected By Atomic radius decreases from left to right within a period. The bond length of a diatomic. We define the atomic radius of a chemical element. Here are the definitions of atomic and ionic radius, the difference between. In simpler terms, it can be defined as something similar to the. One proton has a greater effect than one. Atomic radius or. What Is Atomic Radius Affected By.
From general.chemistrysteps.com
Atomic Radius Chemistry Steps What Is Atomic Radius Affected By The size of the atoms of. This is caused by the increase in the number of protons and electrons across a period. The atomic radius of atoms generally. We define the atomic radius of a chemical element. Atomic radius decreases from left to right within a period. Atomic radius and ionic radius are two of the most common atom size. What Is Atomic Radius Affected By.
From ar.inspiredpencil.com
Measuring Atomic Radius What Is Atomic Radius Affected By Atomic radius decreases from left to right within a period. The atomic radius of atoms generally. The size of the atoms of. We define the atomic radius of a chemical element. For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal: Atomic radius is generally stated as being the. What Is Atomic Radius Affected By.
From kunduz.com
[ANSWERED] radius 1 the largest atomic radius 3 the smallest atomic What Is Atomic Radius Affected By The bond length of a diatomic. This is caused by the increase in the number of protons and electrons across a period. For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal: One proton has a greater effect than one. Atomic radius decreases from left to right within a. What Is Atomic Radius Affected By.
From periodictableguide.com
Get the Periodic table with Atomic radius values (Img+Chart) What Is Atomic Radius Affected By The atomic radius of atoms generally. Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. The bond length of a diatomic. Atomic radius and ionic radius are two of the most common atom size measurements. Bond length from atomic radii. This is caused by the increase in the number of protons and. What Is Atomic Radius Affected By.
From www.slideserve.com
PPT Unit 2 20142015 PowerPoint Presentation, free download ID What Is Atomic Radius Affected By Atomic radius or atomic radii is the total distance from the nucleus of an atom to the outermost orbital of its electron. For metals, the atomic radius is defined as half the distance between two neighboring atoms in a crystal of the metal: Here are the definitions of atomic and ionic radius, the difference between. Bond length from atomic radii.. What Is Atomic Radius Affected By.
From stock.adobe.com
Atomic Size and Atomic Radius. The radius of a carbon atom. Atomic What Is Atomic Radius Affected By Atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic radius decreases from left to right within a period. One proton has a greater effect than one. The size of the atoms of. Atomic radius is generally stated as being the total distance from an atom’s nucleus to the outermost orbital of. What Is Atomic Radius Affected By.