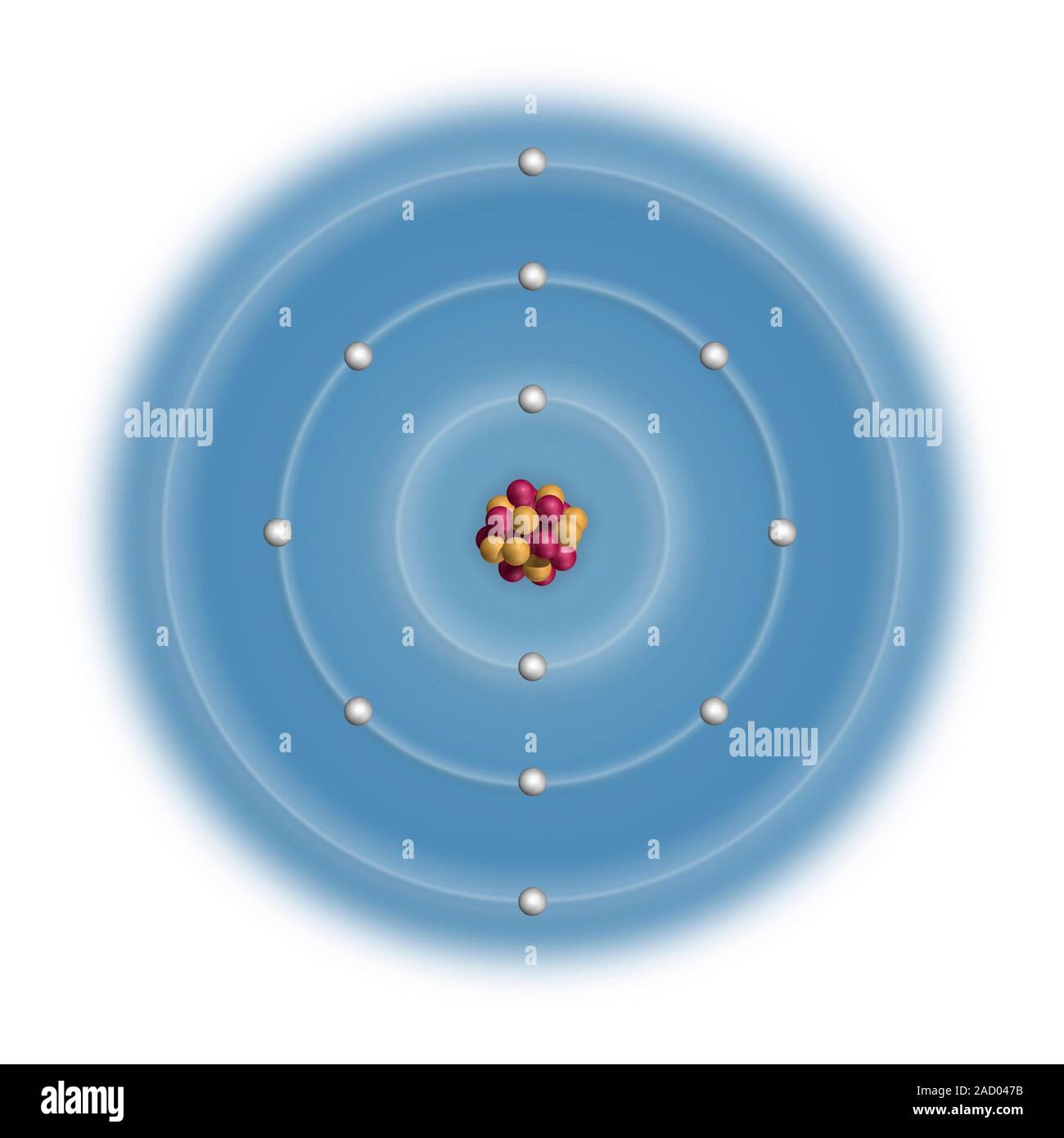
Electron Configuration Of Magnesium 2+ . The shorthand electron configuration (or. Okay, so we are given these answer choices,. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. 119 rows electron configuration chart of all elements is mentioned in the table below. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Since 1s can only hold two electrons the. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. It is not similar to ar. Magnesium has 12 electrons arranged in three energy levels. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). In this video we will write the electron configuration for mg 2+, the magnesium ion.
from www.alamy.com
Since 1s can only hold two electrons the. It is not similar to ar. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. 119 rows electron configuration chart of all elements is mentioned in the table below. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Okay, so we are given these answer choices,. The shorthand electron configuration (or. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+.
Magnesium (Mg). Diagram of the nuclear composition and electron
Electron Configuration Of Magnesium 2+ The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). The shorthand electron configuration (or. Magnesium has 12 electrons arranged in three energy levels. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Okay, so we are given these answer choices,. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. In this video we will write the electron configuration for mg 2+, the magnesium ion. 119 rows electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². It is not similar to ar.
From valenceelectrons.com
Magnesium(Mg) electron configuration and orbital diagram Electron Configuration Of Magnesium 2+ It is not similar to ar. 119 rows electron configuration chart of all elements is mentioned in the table below. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the. The shorthand electron configuration (or. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s².. Electron Configuration Of Magnesium 2+.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition, electron Electron Configuration Of Magnesium 2+ Since 1s can only hold two electrons the. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. Magnesium has 12 electrons arranged in three energy levels. The shorthand electron configuration (or. Use the periodic table to predict the valence electron configuration of all the. Electron Configuration Of Magnesium 2+.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Electron Configuration Of Magnesium 2+ In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). It is not similar to. Electron Configuration Of Magnesium 2+.
From www.coursehero.com
[Solved] Write the short form electron configuration for magnesium Electron Configuration Of Magnesium 2+ 119 rows electron configuration chart of all elements is mentioned in the table below. It is not similar to ar. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium has 12 electrons arranged in three energy levels. In this video we will write the electron configuration for mg 2+, the magnesium. Electron Configuration Of Magnesium 2+.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Electron Configuration Of Magnesium 2+ Since 1s can only hold two electrons the. Okay, so we are given these answer choices,. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². 119 rows electron configuration chart of all elements is mentioned in the table below. Magnesium has 12 electrons arranged in three energy levels. The electron configuration of the magnesium ion mg2+ is similar to. Electron Configuration Of Magnesium 2+.
From www.alamy.com
Magnesium (Mg). Diagram of the nuclear composition and electron Electron Configuration Of Magnesium 2+ In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium has 12 electrons arranged in three energy levels. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). The shorthand electron configuration (or. The electron configuration of mg2+. Electron Configuration Of Magnesium 2+.
From wirelistcollegium.z14.web.core.windows.net
Magnesium Orbital Diagram Electron Configuration Of Magnesium 2+ Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). 119 rows electron configuration chart of all elements is mentioned in the table below. It is not similar to ar. In this video we will write the electron configuration for mg 2+, the magnesium ion. Since. Electron Configuration Of Magnesium 2+.
From joizkfdrp.blob.core.windows.net
Magnesium Ion Aufbau Diagram at Katherine Cortez blog Electron Configuration Of Magnesium 2+ The shorthand electron configuration (or. Since 1s can only hold two electrons the. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. 119 rows electron configuration chart of all elements is mentioned in the table below. Use the periodic table to predict the valence electron configuration of all the elements of group. Electron Configuration Of Magnesium 2+.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Electron Configuration Of Magnesium 2+ Since 1s can only hold two electrons the. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). The shorthand electron configuration (or. Okay, so we are given these answer choices,. It is not similar to ar. The electron configuration of the magnesium ion mg2+ is. Electron Configuration Of Magnesium 2+.
From enginedatanichered.z21.web.core.windows.net
Magnesium Atom Diagram Electron Configuration Of Magnesium 2+ The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Since 1s can only hold two electrons the. Magnesium has 12 electrons arranged in three energy levels. 119 rows electron configuration chart of all elements is mentioned in the table below. The electron configuration of magnesium is. Electron Configuration Of Magnesium 2+.
From www.pearson.com
Show the complete orbital diagram of magnesium. Channels for Pearson+ Electron Configuration Of Magnesium 2+ It is not similar to ar. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or. Magnesium has 12 electrons arranged in three energy levels. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has. Electron Configuration Of Magnesium 2+.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Electron Configuration Of Magnesium 2+ In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. It is not similar to ar. Magnesium has 12 electrons arranged in three energy levels. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). The electron configuration of. Electron Configuration Of Magnesium 2+.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Electron Configuration Of Magnesium 2+ The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Since 1s can only hold two electrons the. In this video we will write the electron configuration for mg 2+, the magnesium ion. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Magnesium has 12. Electron Configuration Of Magnesium 2+.
From sciencenotes.org
Magnesium Atom Science Notes and Projects Electron Configuration Of Magnesium 2+ Okay, so we are given these answer choices,. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). It is not similar to. Electron Configuration Of Magnesium 2+.
From www.toppr.com
Write the electron dot structure of magnesium and oxygen. Electron Configuration Of Magnesium 2+ Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In this video we will write the electron configuration for mg 2+, the. Electron Configuration Of Magnesium 2+.
From mavink.com
Draw The Orbital Diagram For Magnesium Electron Configuration Of Magnesium 2+ 119 rows electron configuration chart of all elements is mentioned in the table below. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Since 1s can only hold two electrons the.. Electron Configuration Of Magnesium 2+.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Electron Configuration Of Magnesium 2+ 119 rows electron configuration chart of all elements is mentioned in the table below. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). It is not similar to ar. The shorthand electron configuration (or. Since 1s can only hold two electrons the. Okay, so we. Electron Configuration Of Magnesium 2+.
From brainly.in
Write lewis dot structure of Mg2+ Brainly.in Electron Configuration Of Magnesium 2+ The shorthand electron configuration (or. Magnesium has 12 electrons arranged in three energy levels. Since 1s can only hold two electrons the. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. In this video we will write the electron configuration for mg 2+, the magnesium ion. 119 rows electron configuration chart of. Electron Configuration Of Magnesium 2+.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Electron Configuration Of Magnesium 2+ 119 rows electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. Okay, so we are given these answer. Electron Configuration Of Magnesium 2+.
From www.shutterstock.com
Magnesium Atom. Diagram Representation Of The Element Magnesium Electron Configuration Of Magnesium 2+ Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Okay, so we are given these answer choices,. In this video we will write the electron configuration for mg 2+, the magnesium ion. Magnesium has 12 electrons arranged in three energy levels. The electron configuration of. Electron Configuration Of Magnesium 2+.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Electron Configuration Of Magnesium 2+ 119 rows electron configuration chart of all elements is mentioned in the table below. It is not similar to ar. Okay, so we are given these answer choices,. Magnesium has 12 electrons arranged in three energy levels. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. Use the periodic table to predict the valence. Electron Configuration Of Magnesium 2+.
From guidedehartsculptures.z21.web.core.windows.net
Magnesium Electric Dot Diagram Electron Configuration Of Magnesium 2+ It is not similar to ar. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Magnesium has 12 electrons arranged in three energy levels. The shorthand electron configuration (or. In this video we will write the electron configuration for mg 2+, the magnesium ion. Since 1s can only hold two electrons the. The electron configuration of the magnesium ion. Electron Configuration Of Magnesium 2+.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Electron Configuration Of Magnesium 2+ Okay, so we are given these answer choices,. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Magnesium has 12 electrons arranged in three energy levels. It is not similar to ar. In this video we will write the electron configuration for mg 2+, the. Electron Configuration Of Magnesium 2+.
From www.pinterest.com
Magnesium, atomic structure Stock Image C018/3693 Science Photo Electron Configuration Of Magnesium 2+ Okay, so we are given these answer choices,. The shorthand electron configuration (or. Magnesium has 12 electrons arranged in three energy levels. In this video we will write the electron configuration for mg 2+, the magnesium ion. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Use the periodic table to predict. Electron Configuration Of Magnesium 2+.
From www.dreamstime.com
Electron of the Element Magnesium Stock Vector Illustration of Electron Configuration Of Magnesium 2+ The shorthand electron configuration (or. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium has 12 electrons arranged in three energy levels. In this video we will write the electron configuration for mg 2+, the magnesium ion. The electron configuration of. Electron Configuration Of Magnesium 2+.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Magnesium 2+ 119 rows electron configuration chart of all elements is mentioned in the table below. It is not similar to ar. Magnesium has 12 electrons arranged in three energy levels. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). The shorthand electron configuration (or. Okay, so we. Electron Configuration Of Magnesium 2+.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Electron Configuration Of Magnesium 2+ The shorthand electron configuration (or. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In this video we will write the electron configuration for mg 2+, the magnesium ion. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of the magnesium ion. Electron Configuration Of Magnesium 2+.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Magnesium 2+ Since 1s can only hold two electrons the. The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The shorthand electron configuration (or. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium has 12 electrons arranged in three energy levels. The electron configuration of the magnesium ion mg2+ is similar to. Electron Configuration Of Magnesium 2+.
From guidemanualeruptivity.z14.web.core.windows.net
Electronic Structure Diagram For Magnesium Electron Configuration Of Magnesium 2+ The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. In this video we will write the electron configuration for mg 2+, the magnesium ion. It is not. Electron Configuration Of Magnesium 2+.
From valenceelectrons.com
Electron Configuration for Magnesium (Mg, Mg2+ ion) Electron Configuration Of Magnesium 2+ The electron configuration of magnesium is 1s² 2s² 2p⁶ 3s². Okay, so we are given these answer choices,. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). Since 1s can only hold two electrons the. Magnesium has 12 electrons arranged in three energy levels. The shorthand. Electron Configuration Of Magnesium 2+.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Magnesium 2+ In this video we will write the electron configuration for mg 2+, the magnesium ion. It is not similar to ar. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne). In writing the electron configuration for magnesium the first two electrons will go in the 1s. Electron Configuration Of Magnesium 2+.
From www.newtondesk.com
magnesium electron configuration Newton Desk Electron Configuration Of Magnesium 2+ In this video we will write the electron configuration for mg 2+, the magnesium ion. Use the periodic table to predict the valence electron configuration of all the elements of group 2 (beryllium, magnesium, calcium, strontium, barium, and radium). Since 1s can only hold two electrons the. 119 rows electron configuration chart of all elements is mentioned in the table. Electron Configuration Of Magnesium 2+.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Magnesium 2+ In this video we will write the electron configuration for mg 2+, the magnesium ion. Since 1s can only hold two electrons the. 119 rows electron configuration chart of all elements is mentioned in the table below. The electron configuration of mg2+ is 1s² 2s² 2p⁶, meaning that it has the same electron configuration as the noble gas neon (ne).. Electron Configuration Of Magnesium 2+.
From www.schoolmykids.com
Magnesium (Mg) Element Information, Facts, Properties, Uses Electron Configuration Of Magnesium 2+ It is not similar to ar. Okay, so we are given these answer choices,. 119 rows electron configuration chart of all elements is mentioned in the table below. The electron configuration of the magnesium ion mg2+ is similar to na+, ne, and al3+. Use the periodic table to predict the valence electron configuration of all the elements of group 2. Electron Configuration Of Magnesium 2+.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Magnesium 2+ Magnesium has 12 electrons arranged in three energy levels. 119 rows electron configuration chart of all elements is mentioned in the table below. Since 1s can only hold two electrons the. In this video we will write the electron configuration for mg 2+, the magnesium ion. It is not similar to ar. Use the periodic table to predict the valence. Electron Configuration Of Magnesium 2+.