Bromide Ion Precipitate . Precipitates can be coloured or. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. A white precipitate indicates the presence of the chloride ion. Chloride ions give a white precipitate of silver chloride. Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ). A solid substance that has been separated from a liquid in a chemical process is called a precipitate. Agno 3 solution is often used in a similar way to test for halide ion. That process is defined as precipitating. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). Dissolve a small quantity of the substance in water. Form, depending on the halide ions present: A cream precipitate indicates the presence of the bromide ion. Identify bromide ion in solution state.
from www.nagwa.com
A cream precipitate indicates the presence of the bromide ion. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ). Chloride ions give a white precipitate of silver chloride. Precipitates can be coloured or. A solid substance that has been separated from a liquid in a chemical process is called a precipitate. Agno 3 solution is often used in a similar way to test for halide ion. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. That process is defined as precipitating. Form, depending on the halide ions present:
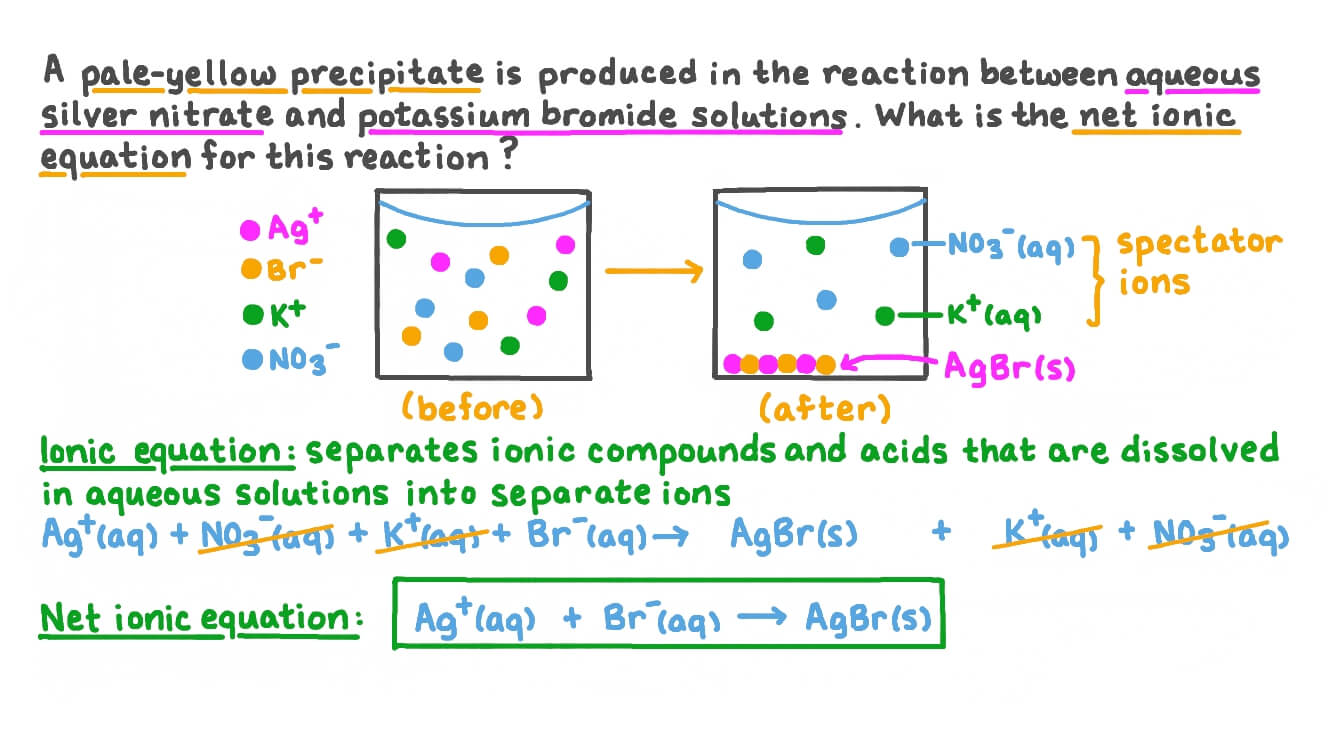
Question Video Writing a Net Ionic Equation for the Reaction between
Bromide Ion Precipitate Form, depending on the halide ions present: Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ). A white precipitate indicates the presence of the chloride ion. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). Chloride ions give a white precipitate of silver chloride. Precipitates can be coloured or. Identify bromide ion in solution state. A cream precipitate indicates the presence of the bromide ion. Dissolve a small quantity of the substance in water. That process is defined as precipitating. Form, depending on the halide ions present: Agno 3 solution is often used in a similar way to test for halide ion. A solid substance that has been separated from a liquid in a chemical process is called a precipitate. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products.
From www.researchgate.net
Correlation between concentration of bromide ion solution and amount of Bromide Ion Precipitate Precipitates can be coloured or. A solid substance that has been separated from a liquid in a chemical process is called a precipitate. A cream precipitate indicates the presence of the bromide ion. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Add lead (ii) acetate ( pb (ch 3 coo) 2. Bromide Ion Precipitate.
From www.numerade.com
SOLVED Will potassium hydroxide and magnesium bromide form a Bromide Ion Precipitate Dissolve a small quantity of the substance in water. A cream precipitate indicates the presence of the bromide ion. Precipitates can be coloured or. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). That process is defined as precipitating. Chloride ions give a white precipitate of silver chloride. Form, depending on the halide ions present: A solid. Bromide Ion Precipitate.
From www.numerade.com
SOLVEDA 0.244 g sample of a compound of phosphorus and bromine, when Bromide Ion Precipitate A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. A solid substance that has been separated from a liquid in a chemical process is called a precipitate. Form, depending on the halide ions present: Agno 3 solution is often used in a similar way to test for halide ion. Identify bromide ion. Bromide Ion Precipitate.
From www.nagwa.com
Question Video Writing a Net Ionic Equation for the Reaction between Bromide Ion Precipitate A cream precipitate indicates the presence of the bromide ion. A white precipitate indicates the presence of the chloride ion. A solid substance that has been separated from a liquid in a chemical process is called a precipitate. Form, depending on the halide ions present: Chloride ions give a white precipitate of silver chloride. A precipitation reaction is one in. Bromide Ion Precipitate.
From ask.modifiyegaraj.com
What Best Describes The Bromide Ion That Forms Asking List Bromide Ion Precipitate A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). Form, depending on the halide ions present: Identify bromide ion in solution state. That process is defined as precipitating. Precipitates can be coloured or. A cream precipitate indicates the presence of. Bromide Ion Precipitate.
From ask.modifiyegaraj.com
What Best Describes The Bromide Ion That Forms Asking List Bromide Ion Precipitate Identify bromide ion in solution state. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). Form, depending on the halide ions present: Chloride ions give a white precipitate of silver chloride. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Add lead (ii) acetate ( pb (ch 3 coo). Bromide Ion Precipitate.
From www.youtube.com
TEST FOR HALIDE IONS (Chloride, Bromide and Iodide ions) 3D animation Bromide Ion Precipitate Dissolve a small quantity of the substance in water. Chloride ions give a white precipitate of silver chloride. That process is defined as precipitating. Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ). A precipitation reaction is one in which dissolved substances react to form one (or more). Bromide Ion Precipitate.
From www.sciencephoto.com
Silver bromide (AgBr) precipitate Stock Image A500/0642 Science Bromide Ion Precipitate Dissolve a small quantity of the substance in water. Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ). A cream precipitate indicates the presence of the bromide ion. A solid substance that has been separated from a liquid in a chemical process is called a precipitate. Form, depending. Bromide Ion Precipitate.
From www.shalom-education.com
Testing for Halide ions GCSE Chemistry Revision Bromide Ion Precipitate Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ). Agno 3 solution is often used in a similar way to test for halide ion. A white precipitate indicates the presence of the chloride ion. Form, depending on the halide ions present: A cream precipitate indicates the presence of. Bromide Ion Precipitate.
From sites.google.com
5.3.2 Tests for ions Ellesmere OCR A level Chemistry Bromide Ion Precipitate Chloride ions give a white precipitate of silver chloride. That process is defined as precipitating. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. A white precipitate indicates the presence of the chloride ion. Agno 3 solution is often used. Bromide Ion Precipitate.
From www.slideserve.com
PPT Precipitate Reactions PowerPoint Presentation, free download ID Bromide Ion Precipitate A white precipitate indicates the presence of the chloride ion. Chloride ions give a white precipitate of silver chloride. Agno 3 solution is often used in a similar way to test for halide ion. That process is defined as precipitating. A cream precipitate indicates the presence of the bromide ion. A solid substance that has been separated from a liquid. Bromide Ion Precipitate.
From ask.modifiyegaraj.com
What Best Describes The Bromide Ion That Forms Asking List Bromide Ion Precipitate Dissolve a small quantity of the substance in water. Identify bromide ion in solution state. Form, depending on the halide ions present: Agno 3 solution is often used in a similar way to test for halide ion. A cream precipitate indicates the presence of the bromide ion. A precipitation reaction is one in which dissolved substances react to form one. Bromide Ion Precipitate.
From www.youtube.com
Sodium bromide YouTube Bromide Ion Precipitate A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Chloride ions give a white precipitate of silver chloride. Precipitates can be coloured or. Identify bromide ion in solution state. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). A cream precipitate indicates the presence of the bromide ion. A. Bromide Ion Precipitate.
From www.chegg.com
Solved The bromide ion concentration in a solution may be Bromide Ion Precipitate Chloride ions give a white precipitate of silver chloride. Identify bromide ion in solution state. Agno 3 solution is often used in a similar way to test for halide ion. A white precipitate indicates the presence of the chloride ion. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. It will give. Bromide Ion Precipitate.
From edu.svet.gob.gt
Bromide Ions And Silver Nitrate edu.svet.gob.gt Bromide Ion Precipitate Form, depending on the halide ions present: Precipitates can be coloured or. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Agno 3 solution is often used in a similar way to test for halide ion. A cream precipitate indicates. Bromide Ion Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Bromide Ion Precipitate A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ). A solid substance that has been separated from a liquid in a chemical process is called a precipitate. Identify bromide ion in. Bromide Ion Precipitate.
From www.chegg.com
Solved does zinc bromide and sodium sulfide precipitate and Bromide Ion Precipitate A solid substance that has been separated from a liquid in a chemical process is called a precipitate. Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ). Precipitates can be coloured or. Chloride ions give a white precipitate of silver chloride. A precipitation reaction is one in which. Bromide Ion Precipitate.
From www.sciencephoto.com
Silver bromide precipitate Stock Image C030/7204 Science Photo Bromide Ion Precipitate It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). That process is defined as precipitating. Chloride ions give a white precipitate of silver chloride. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Form, depending on the halide ions present: Agno 3 solution is often used in a similar. Bromide Ion Precipitate.
From www.numerade.com
draw models to represent the formation of the positive calcium ion and Bromide Ion Precipitate That process is defined as precipitating. A solid substance that has been separated from a liquid in a chemical process is called a precipitate. Precipitates can be coloured or. Agno 3 solution is often used in a similar way to test for halide ion. Chloride ions give a white precipitate of silver chloride. Form, depending on the halide ions present:. Bromide Ion Precipitate.
From www.grainger.com
7758023, 119, Potassium Bromide, Crystal, Reagent, ACS 6MMU1P1220 Bromide Ion Precipitate That process is defined as precipitating. Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ). Chloride ions give a white precipitate of silver chloride. Precipitates can be coloured or. A cream precipitate indicates the presence of the bromide ion. Agno 3 solution is often used in a similar. Bromide Ion Precipitate.
From www.numerade.com
A sample of 0.3220 g of an ionic compound containing the bromide ion Bromide Ion Precipitate It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). A solid substance that has been separated from a liquid in a chemical process is called a precipitate. A white precipitate indicates the presence of the chloride ion. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. A cream precipitate. Bromide Ion Precipitate.
From www.numerade.com
SOLVED Oxalate ion, Permanganate ion, Phosphate ion, Hydrogen Bromide Ion Precipitate Chloride ions give a white precipitate of silver chloride. Identify bromide ion in solution state. Precipitates can be coloured or. Dissolve a small quantity of the substance in water. A cream precipitate indicates the presence of the bromide ion. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). A solid substance that has been separated from a. Bromide Ion Precipitate.
From www.youtube.com
Br Electron Configuration (Bromide Ion) YouTube Bromide Ion Precipitate Identify bromide ion in solution state. Chloride ions give a white precipitate of silver chloride. Precipitates can be coloured or. Dissolve a small quantity of the substance in water. A cream precipitate indicates the presence of the bromide ion. Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ).. Bromide Ion Precipitate.
From www.numerade.com
SOLVED Calculate the mass of the precipitate formed when 300.mLof 3.25 Bromide Ion Precipitate Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ). Agno 3 solution is often used in a similar way to test for halide ion. A cream precipitate indicates the presence of the bromide ion. Dissolve a small quantity of the substance in water. A white precipitate indicates the. Bromide Ion Precipitate.
From pubs.acs.org
The Multiple Role of Bromide Ion in PPCPs Degradation under UV/Chlorine Bromide Ion Precipitate Identify bromide ion in solution state. Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate ( pb (no 3) 2 ). Dissolve a small quantity of the substance in water. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). A precipitation reaction is one in which dissolved substances react to form. Bromide Ion Precipitate.
From www.numerade.com
What is the difference between (a) a bromine atom, (b) a bromine Bromide Ion Precipitate Form, depending on the halide ions present: Precipitates can be coloured or. Agno 3 solution is often used in a similar way to test for halide ion. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. A white precipitate indicates the presence of the chloride ion. That process is defined as precipitating.. Bromide Ion Precipitate.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记2.3.4 Halide Ion Reactions翰林国际教育 Bromide Ion Precipitate Identify bromide ion in solution state. Form, depending on the halide ions present: Dissolve a small quantity of the substance in water. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Chloride ions give a white precipitate of silver chloride. A solid substance that has been separated from a liquid in a. Bromide Ion Precipitate.
From brainly.com
PLEASE HELP Which particle represents the size of the bromide ion Bromide Ion Precipitate Agno 3 solution is often used in a similar way to test for halide ion. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). A cream precipitate indicates the presence of the bromide ion. Form, depending on the halide ions present: Add lead (ii) acetate ( pb (ch 3 coo) 2 ) or lead (ii) nitrate (. Bromide Ion Precipitate.
From www.shutterstock.com
Sodium Bromide Properties Chemical Compound Structure Stock Vector Bromide Ion Precipitate A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Identify bromide ion in solution state. Precipitates can be coloured or. A cream precipitate indicates the presence of the bromide ion. Chloride ions give a white precipitate of silver chloride. A white precipitate indicates the presence of the chloride ion. Add lead (ii). Bromide Ion Precipitate.
From www.numerade.com
SOLVED Sn(s) HCI(aq) results in oxidation of hydrogen ion. formation Bromide Ion Precipitate Agno 3 solution is often used in a similar way to test for halide ion. A solid substance that has been separated from a liquid in a chemical process is called a precipitate. That process is defined as precipitating. A cream precipitate indicates the presence of the bromide ion. A white precipitate indicates the presence of the chloride ion. Chloride. Bromide Ion Precipitate.
From www.sciencephoto.com
Silver bromide (AgBr) precipitate Stock Image A500/0640 Science Bromide Ion Precipitate Identify bromide ion in solution state. Form, depending on the halide ions present: Precipitates can be coloured or. A solid substance that has been separated from a liquid in a chemical process is called a precipitate. That process is defined as precipitating. Dissolve a small quantity of the substance in water. Chloride ions give a white precipitate of silver chloride.. Bromide Ion Precipitate.
From www.sciencephoto.com
Lead bromide precipitate Stock Image C030/7209 Science Photo Library Bromide Ion Precipitate Precipitates can be coloured or. A white precipitate indicates the presence of the chloride ion. Agno 3 solution is often used in a similar way to test for halide ion. That process is defined as precipitating. Identify bromide ion in solution state. A cream precipitate indicates the presence of the bromide ion. Chloride ions give a white precipitate of silver. Bromide Ion Precipitate.
From amudu-gowripalan.blogspot.com
amudu Magical precipitate of Chemistry Bromide Ion Precipitate Identify bromide ion in solution state. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Form, depending on the halide ions present: A cream precipitate indicates the presence of the bromide ion. Precipitates can be coloured or. Chloride ions give a white precipitate of silver chloride. That process is defined as precipitating.. Bromide Ion Precipitate.
From www.numerade.com
SOLVED 1.A sample of 0.3220 g of an ionic compound containing the Bromide Ion Precipitate Agno 3 solution is often used in a similar way to test for halide ion. It will give white (cream) precipitate of lead (ii) bromide (pbbr 2). Dissolve a small quantity of the substance in water. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Chloride ions give a white precipitate of. Bromide Ion Precipitate.
From www.toppr.com
Solubility product of silver bromide is 5.0 × 10^13 . The quantity of Bromide Ion Precipitate Agno 3 solution is often used in a similar way to test for halide ion. Form, depending on the halide ions present: A white precipitate indicates the presence of the chloride ion. A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Add lead (ii) acetate ( pb (ch 3 coo) 2 ). Bromide Ion Precipitate.