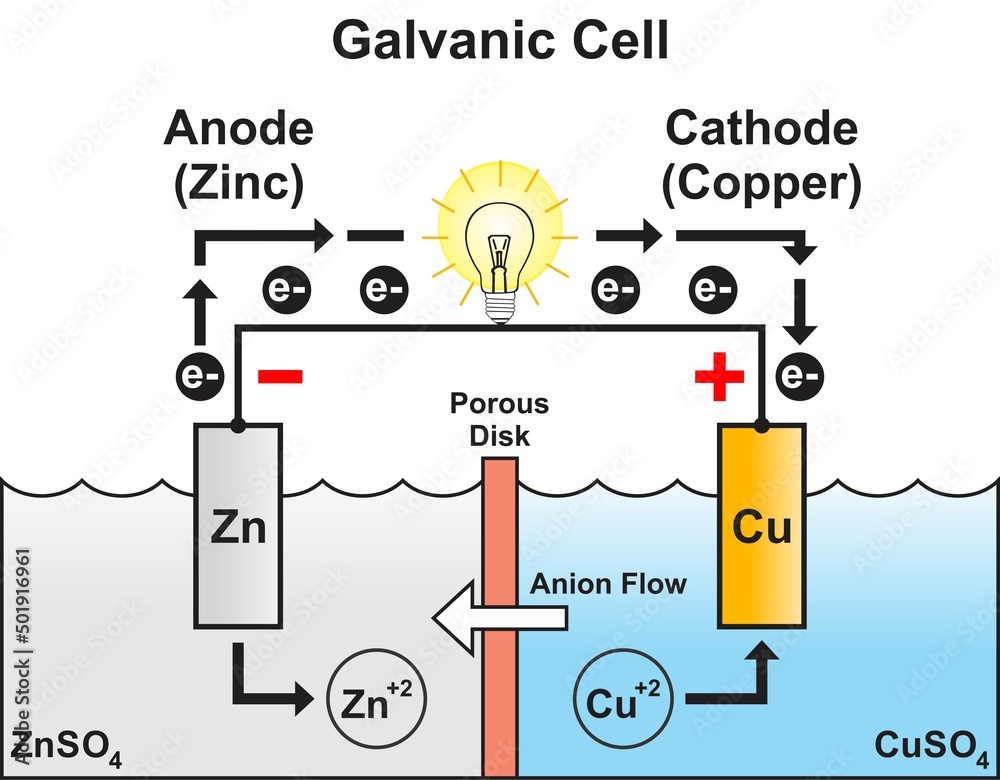
Cell Using Zinc And Copper . The daniell cell consists of two electrodes of dissimilar. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. As the reaction goes forward, metallic copper can. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. Answer from the information given in.
from stock.adobe.com
Answer from the information given in. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. The daniell cell consists of two electrodes of dissimilar. As the reaction goes forward, metallic copper can. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc.
Galvanic voltaic cell infographic diagram battery part structure
Cell Using Zinc And Copper Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. The daniell cell consists of two electrodes of dissimilar. Answer from the information given in. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. As the reaction goes forward, metallic copper can. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Cell Using Zinc And Copper Answer from the information given in. As the reaction goes forward, metallic copper can. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. This 2:54 minute video shows the spontaneous reaction between. Cell Using Zinc And Copper.
From www.slideserve.com
PPT Electrochemical Cells PowerPoint Presentation, free download ID Cell Using Zinc And Copper This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. Use cell notation to describe the galvanic cell where copper(ii). Cell Using Zinc And Copper.
From www.youtube.com
ZincCopper Electrochemical Cell Demonstration YouTube Cell Using Zinc And Copper Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. The daniell cell consists of two electrodes of dissimilar. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. This 2:54 minute video shows the spontaneous reaction. Cell Using Zinc And Copper.
From www.slideserve.com
PPT Chapter 22 Electrochemistry PowerPoint Presentation, free Cell Using Zinc And Copper This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. As the reaction goes forward, metallic copper can. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to. Cell Using Zinc And Copper.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation ID1195562 Cell Using Zinc And Copper Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. As the reaction goes forward, metallic copper can. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. The daniell cell consists of two electrodes of dissimilar. This reaction can be observed by. Cell Using Zinc And Copper.
From www.youtube.com
Electrochemical cell Zn and Cu YouTube Cell Using Zinc And Copper An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. As the reaction goes forward, metallic copper can. The daniell cell consists of two electrodes of dissimilar. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Answer from. Cell Using Zinc And Copper.
From www1.chem.umn.edu
CopperZinc Galvanic Cell Cell Using Zinc And Copper This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. The daniell cell consists of. Cell Using Zinc And Copper.
From socratic.org
What is a voltaic cell? + Example Cell Using Zinc And Copper An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. The daniell cell consists of two electrodes of dissimilar. As the reaction. Cell Using Zinc And Copper.
From www.alamy.com
Copper zinc cell Cut Out Stock Images & Pictures Alamy Cell Using Zinc And Copper Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Answer from the information given in. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. As the reaction goes forward, metallic copper can. The daniell cell. Cell Using Zinc And Copper.
From www.researchgate.net
(PDF) Better Batteries Through Electrochemistry Cell Using Zinc And Copper A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. Answer from the information given in.. Cell Using Zinc And Copper.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure Cell Using Zinc And Copper Answer from the information given in. The daniell cell consists of two electrodes of dissimilar. As the reaction goes forward, metallic copper can. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. This. Cell Using Zinc And Copper.
From chem.libretexts.org
20.4 Cell Potential Under Standard Conditions Chemistry LibreTexts Cell Using Zinc And Copper The daniell cell consists of two electrodes of dissimilar. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper. Cell Using Zinc And Copper.
From www.slideserve.com
PPT Chapter 20 PowerPoint Presentation, free download ID6976551 Cell Using Zinc And Copper As the reaction goes forward, metallic copper can. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper. Cell Using Zinc And Copper.
From www.embibe.com
How can you electroplate an iron nail with copper Explain with the help Cell Using Zinc And Copper As the reaction goes forward, metallic copper can. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. Answer from the information. Cell Using Zinc And Copper.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Cell Using Zinc And Copper Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. Answer from the information given in. The daniell cell consists of two electrodes of dissimilar. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. This 2:54 minute video. Cell Using Zinc And Copper.
From www.alamy.com
Daniell element galvanic cell with zinc and copper Stock Photo Cell Using Zinc And Copper Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. Answer from the information given in. As the reaction goes forward, metallic copper can. An extremely important class of oxidation and reduction reactions. Cell Using Zinc And Copper.
From www.chegg.com
Solved The Zinccopper galvanic cell shown below is Cell Using Zinc And Copper As the reaction goes forward, metallic copper can. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. An extremely important class of oxidation and reduction reactions are. Cell Using Zinc And Copper.
From www.youtube.com
Standard ZincCopper Voltaic Cell with Salt Bridge YouTube Cell Using Zinc And Copper This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. As the reaction goes. Cell Using Zinc And Copper.
From classnotes.org.in
Extraction of Copper and Zinc Chemistry, Class 12, General Principles Cell Using Zinc And Copper As the reaction goes forward, metallic copper can. Answer from the information given in. The daniell cell consists of two electrodes of dissimilar. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate.. Cell Using Zinc And Copper.
From www.slideserve.com
PPT OxidationReduction Reactions PowerPoint Presentation, free Cell Using Zinc And Copper The daniell cell consists of two electrodes of dissimilar. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal. Cell Using Zinc And Copper.
From psu.pb.unizin.org
17.7 Electrolysis Chemistry 112 Chapters 1217 of OpenStax General Cell Using Zinc And Copper This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. Answer from the information given in. As the reaction goes. Cell Using Zinc And Copper.
From 2012books.lardbucket.org
Electrochemistry Cell Using Zinc And Copper A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. Use cell notation to describe the galvanic cell where copper(ii). Cell Using Zinc And Copper.
From www.youtube.com
Determination of Standard Electrode potential of Zinc and Copper YouTube Cell Using Zinc And Copper The daniell cell consists of two electrodes of dissimilar. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. Answer from the information given in. Use cell notation to describe the galvanic cell. Cell Using Zinc And Copper.
From www.vrogue.co
Solved Draw A Simple Diagram Of A Galvanic Cell Using vrogue.co Cell Using Zinc And Copper This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. As the reaction goes forward, metallic copper can. The daniell cell consists of two electrodes of dissimilar. Answer from the information given in. Use cell notation to describe. Cell Using Zinc And Copper.
From byjus.com
Daniell Cell Definition, Construction & Working with Cell Reactions Cell Using Zinc And Copper The daniell cell consists of two electrodes of dissimilar. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. Answer from the information given in. As the reaction goes forward, metallic copper can. Use cell notation to describe. Cell Using Zinc And Copper.
From mavink.com
Zinc Copper Galvanic Cell Cell Using Zinc And Copper This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. Answer from the information given in. As the reaction goes forward, metallic copper can. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. The daniell cell consists of two electrodes of dissimilar. An extremely important class of oxidation. Cell Using Zinc And Copper.
From courses.lumenlearning.com
Galvanic Cells Chemistry Cell Using Zinc And Copper The daniell cell consists of two electrodes of dissimilar. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. As the reaction goes forward, metallic copper can. This reaction. Cell Using Zinc And Copper.
From brainly.in
label the parts of simple electrical cell (diagram given above Cell Using Zinc And Copper As the reaction goes forward, metallic copper can. The daniell cell consists of two electrodes of dissimilar. Answer from the information given in. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. This 2:54. Cell Using Zinc And Copper.
From www.slideserve.com
PPT What is an Electrochemical Cell? PowerPoint Presentation, free Cell Using Zinc And Copper This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. As the reaction goes forward, metallic copper can. An extremely. Cell Using Zinc And Copper.
From 2012books.lardbucket.org
Describing Electrochemical Cells Cell Using Zinc And Copper The daniell cell consists of two electrodes of dissimilar. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. Answer from the information given in. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. This reaction can. Cell Using Zinc And Copper.
From colouremployer8.gitlab.io
Marvelous Copper Zinc Battery Reaction Cie Chemistry A Level Syllabus Cell Using Zinc And Copper This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. As the reaction goes forward, metallic. Cell Using Zinc And Copper.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Cell Using Zinc And Copper As the reaction goes forward, metallic copper can. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. An extremely important class of oxidation and reduction reactions are. Cell Using Zinc And Copper.
From courses.lumenlearning.com
Galvanic Cells Chemistry Cell Using Zinc And Copper Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in. Answer from the information given in. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc.. Cell Using Zinc And Copper.
From www.youtube.com
COMPONENTS OF A ZINC COPPER VOLTAIC CELL YouTube Cell Using Zinc And Copper A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. The daniell cell consists of two electrodes of dissimilar. This 2:54 minute video shows the spontaneous reaction between copper ions and zinc. This reaction can be observed by placing a strip of zinc metal into a solution of copper (ii) sulfate. Use. Cell Using Zinc And Copper.
From ar.inspiredpencil.com
Copper Electrolytic Cell Cell Using Zinc And Copper Answer from the information given in. Use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc metal is oxidized to zinc ions. The daniell cell consists of two electrodes of dissimilar. A daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. This 2:54 minute. Cell Using Zinc And Copper.