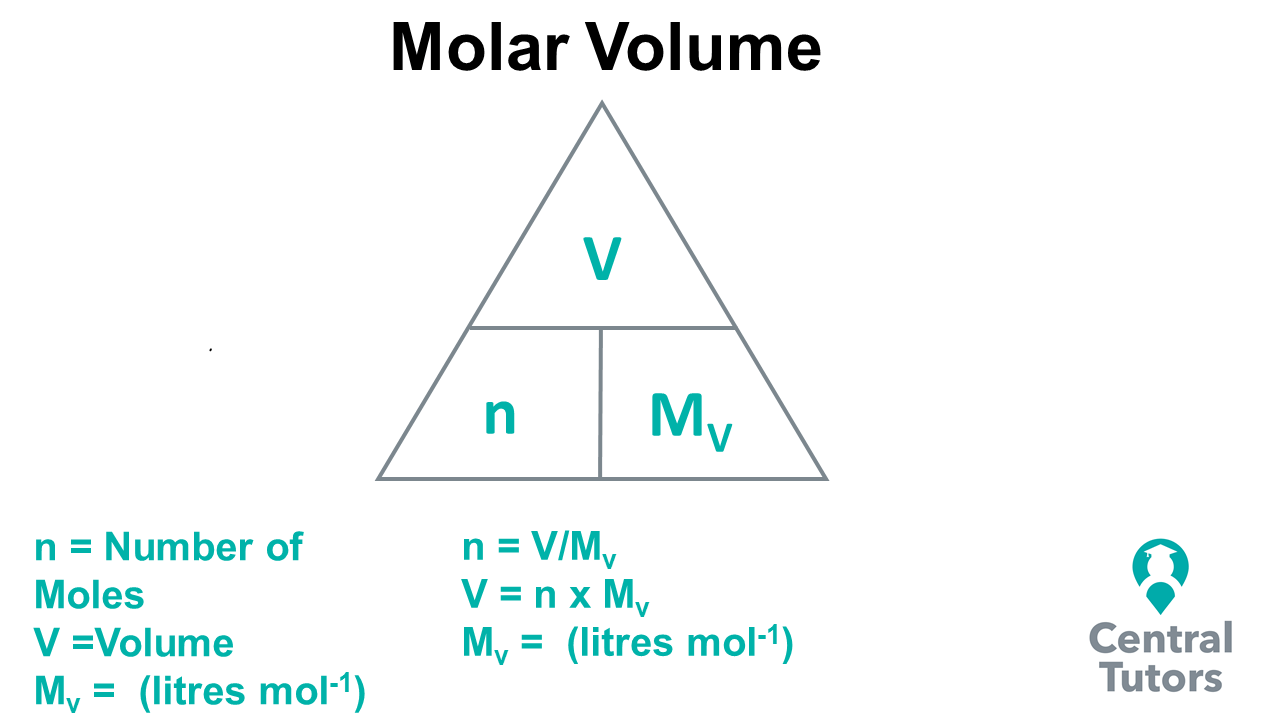
Triangle Equation For Moles . Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o + 2 × mass of. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. See examples of how to use the. Calculations involving conversions between moles of a material and the mass of that material are described. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar.
from centraltutors.co.uk
See examples of how to use the. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Calculations involving conversions between moles of a material and the mass of that material are described. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o + 2 × mass of. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar.
How to solve Mole Calculations using Molar Volume Central Tutors
Triangle Equation For Moles Calculations involving conversions between moles of a material and the mass of that material are described. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o + 2 × mass of. Calculations involving conversions between moles of a material and the mass of that material are described. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. See examples of how to use the. Learn how to calculate the concentration of solutions using moles, volume and formula triangles.
From www.reddit.com
Chemistry giant formula triangle r/GCSE Triangle Equation For Moles See examples of how to use the. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. Calculate the moles of water using its mass and molar mass m. Triangle Equation For Moles.
From sites.google.com
2Mole Triangle Lufkin Chemistry Triangle Equation For Moles See examples of how to use the. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Calculations involving conversions between moles of a material and the mass of that material are described. Calculate the moles. Triangle Equation For Moles.
From www.shutterstock.com
Mole Formula Triangle Pyramid Isolated On Stock Vector (Royalty Free) 1990476140 Triangle Equation For Moles Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula.. Triangle Equation For Moles.
From www.thesciencehive.co.uk
Chemical formulae, equations and calculations GCSE — the science sauce Triangle Equation For Moles Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. Learn how to calculate the mass, number. Triangle Equation For Moles.
From pt.slideshare.net
Formula triangles for moles Triangle Equation For Moles Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o + 2 × mass of. See examples of how to use the.. Triangle Equation For Moles.
From www.youtube.com
Mole Triangles.wmv YouTube Triangle Equation For Moles Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m. Triangle Equation For Moles.
From iteachly.com
Mole Conversion Worksheet and Activity ⋆ Triangle Equation For Moles Learn how to calculate the concentration of solutions using moles, volume and formula triangles. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. See examples of how to use the. Calculations. Triangle Equation For Moles.
From www.savemyexams.com
Moles, Mass & RFM Edexcel IGCSE Chemistry Revision Notes 2019 Triangle Equation For Moles Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar.. Triangle Equation For Moles.
From www.alamyimages.fr
Conception scientifique du triangle de formule de Mole. Relation entre Moles, masse et masse Triangle Equation For Moles Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Calculate the moles of water using its. Triangle Equation For Moles.
From www.istockphoto.com
Diagram Of The Mole Formula Triangle Stock Illustration Download Image Now Abstract, Atom Triangle Equation For Moles See examples of how to use the. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. Calculate the moles of water using its mass and molar mass m =. Triangle Equation For Moles.
From www.tes.com
Moles GCSE 91 Higher Teaching Resources Triangle Equation For Moles Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of. Triangle Equation For Moles.
From www.alamy.com
Scientific Designing of The Mole And Molar Volume Formula Triangle. Relationship Between Moles Triangle Equation For Moles It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. See examples of how to use the. Calculate the moles of water using its mass and molar mass m =. Triangle Equation For Moles.
From www.redbubble.com
"Formula Triangles (Moles) Alternate" Poster for Sale by ChemisTees Redbubble Triangle Equation For Moles Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o + 2 × mass of. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Learn how to calculate the molarity of a solution. Triangle Equation For Moles.
From www.youtube.com
Simple Mole Calculations YouTube Triangle Equation For Moles Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. See. Triangle Equation For Moles.
From www.slideserve.com
PPT Chemistry Calculations PowerPoint Presentation, free download ID899752 Triangle Equation For Moles Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o + 2 × mass of. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. See examples of how to use the. It. Triangle Equation For Moles.
From www.slideshare.net
Formula triangles for moles Triangle Equation For Moles See examples of how to use the. Calculations involving conversions between moles of a material and the mass of that material are described. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. It is more useful to a chemist to express concentration in terms of moles per unit. Triangle Equation For Moles.
From www.slideserve.com
PPT Calculations in Chemistry PowerPoint Presentation, free download ID5922821 Triangle Equation For Moles Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o + 2 × mass of. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. Calculations involving conversions between moles of a material. Triangle Equation For Moles.
From centraltutors.co.uk
How to solve Mole Calculations using Molar Volume Central Tutors Triangle Equation For Moles Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. See examples of how to use the. Learn how to calculate the molarity of a solution using the formula m. Triangle Equation For Moles.
From www.shutterstock.com
Mole Triangle Formula Chemistry Vector Illustration Stock Vector (Royalty Free) 2158380453 Triangle Equation For Moles See examples of how to use the. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram. Triangle Equation For Moles.
From www.thesciencehive.co.uk
Rate of Reaction (AQA) — the science sauce Triangle Equation For Moles Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. Calculations involving conversions between moles of a material and the mass of that material are described. See examples of how to use the. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Learn how to calculate. Triangle Equation For Moles.
From stock.adobe.com
Scientific Designing of The Mole And Molar Volume Formula Triangle. Relationship Between Moles Triangle Equation For Moles Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o + 2 × mass of. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. Learn how to calculate the mass, number of. Triangle Equation For Moles.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.19 carry out mole calculations using relative atomic mass (Ar Triangle Equation For Moles See examples of how to use the. Calculations involving conversions between moles of a material and the mass of that material are described. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Learn how. Triangle Equation For Moles.
From mavink.com
Moles Triangle Triangle Equation For Moles Calculations involving conversions between moles of a material and the mass of that material are described. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. Learn how to. Triangle Equation For Moles.
From www.dreamstime.com
The Mole Formula Triangle Relationship Stock Illustration Illustration of atom, molar 285349195 Triangle Equation For Moles Calculations involving conversions between moles of a material and the mass of that material are described. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Calculate the moles of. Triangle Equation For Moles.
From syatillakmk.blogspot.com
SimplyChemistry C3 MOLEMASSNO.PARTICLE CONVERSIONS Triangle Equation For Moles Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. See examples of how to use. Triangle Equation For Moles.
From www.istockphoto.com
Diagram Of The Mole Formula Triangle Stock Illustration Download Image Now Abstract, Atom Triangle Equation For Moles Calculations involving conversions between moles of a material and the mass of that material are described. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. Learn how to calculate the mass, number of moles, concentration or. Triangle Equation For Moles.
From www.redbubble.com
"Formula Triangles (Moles)" Poster for Sale by ChemisTees Redbubble Triangle Equation For Moles Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. Calculate the moles of water. Triangle Equation For Moles.
From learningkurugon1.z22.web.core.windows.net
How To Work Out Moles Using Volume Triangle Equation For Moles Learn how to use formula triangles to calculate the mass, number of moles, concentration or volume of a substance. Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. See examples of how to use the. Learn how to calculate the concentration of solutions using moles, volume and formula. Triangle Equation For Moles.
From www.tes.com
AS Chemistry The Mole and The Avogadro Constant Teaching Resources Triangle Equation For Moles Calculations involving conversions between moles of a material and the mass of that material are described. Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o + 2 × mass of. Learn how to use formula triangles to calculate the mass, number of. Triangle Equation For Moles.
From www.slideserve.com
PPT Mole Calculations PowerPoint Presentation, free download ID902714 Triangle Equation For Moles See examples of how to use the. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o + 2 × mass of. Learn how to calculate the mass, number. Triangle Equation For Moles.
From www.slideshare.net
Formula triangles for moles Triangle Equation For Moles Learn how to calculate the mass, number of moles, concentration or volume of a substance using formula triangles and gram formula. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o. Triangle Equation For Moles.
From www.youtube.com
Finding Moles by Ratio Cambridge IGCSE/O level Chemistry 0620/0971/5070 Lesson 28 part a YouTube Triangle Equation For Moles Calculations involving conversions between moles of a material and the mass of that material are described. It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. See examples of how to. Triangle Equation For Moles.
From www.scribd.com
Mole Triangle PDF Triangle Equation For Moles Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o + 2 × mass of. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. It is more useful to a chemist to express concentration in terms of moles. Triangle Equation For Moles.
From www.ncl.ac.uk
Numeracy, Maths and Statistics Academic Skills Kit Triangle Equation For Moles Learn how to calculate the concentration of solutions using moles, volume and formula triangles. See examples of how to use the. Learn how to calculate the molarity of a solution using the formula m = nsubstance/vsolution, where n is the molar. Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2. Triangle Equation For Moles.
From stock.adobe.com
Scientific Designing of The Mole Formula Triangle. Relationship Between Moles, Mass And Molar Triangle Equation For Moles It is more useful to a chemist to express concentration in terms of moles per unit volume rather than. Learn how to calculate the concentration of solutions using moles, volume and formula triangles. Calculate the moles of water using its mass and molar mass m = relative molecular mass of h 2 o m = 1 × mass of o. Triangle Equation For Moles.