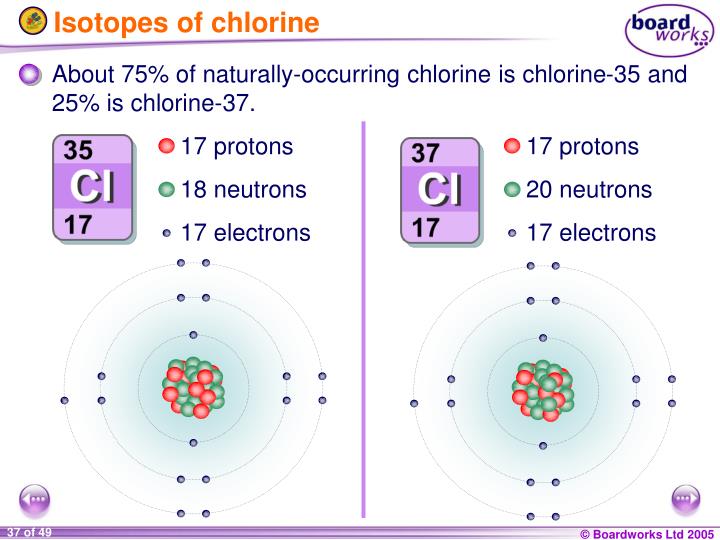
Chlorine Cl Neutron . Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Only two chlorine isotopes exist. It has an atomic weight of 35.450 and a mass. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Among the elements, it has the highest electron affinity and. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. It is an extremely reactive element and a strong oxidising agent:
from brokeasshome.com
It is an extremely reactive element and a strong oxidising agent: Only two chlorine isotopes exist. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Among the elements, it has the highest electron affinity and. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. It has an atomic weight of 35.450 and a mass. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons.
Chlorine Periodic Table Protons Neutrons Electrons
Chlorine Cl Neutron 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Among the elements, it has the highest electron affinity and. It has an atomic weight of 35.450 and a mass. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. It is an extremely reactive element and a strong oxidising agent: Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. Only two chlorine isotopes exist. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Cl Neutron 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. It is an extremely reactive element and a strong oxidising agent: All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. It has an atomic weight of 35.450 and a mass. Naturally occurring chlorine. Chlorine Cl Neutron.
From slideplayer.com
Cl. Chlorine Atomic Atomic Symbol Atomic Mass of protons of electrons of neutrons State Chlorine Cl Neutron 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. It has an atomic weight of 35.450 and a mass. Chlorine is the 17th element in the periodic table and has. Chlorine Cl Neutron.
From www.sciencephoto.com
Isotopes of chlorine, illustration Stock Image C028/6461 Science Photo Library Chlorine Cl Neutron All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. 53 rows cl by neutron capture is an inevitable consequence of using natural. Chlorine Cl Neutron.
From elchoroukhost.net
Chlorine Periodic Table Protons Neutrons Electrons Elcho Table Chlorine Cl Neutron Only two chlorine isotopes exist. It has an atomic weight of 35.450 and a mass. It is an extremely reactive element and a strong oxidising agent: All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 53 rows cl by neutron capture is an. Chlorine Cl Neutron.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Cl Neutron Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. It is an extremely reactive element and a strong oxidising agent: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of. Chlorine Cl Neutron.
From telgurus.co.uk
How to calculate number of neutrons, protons and electrons in Chlorine atom? Chlorine Cl Neutron All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Among the elements, it has the highest electron affinity and. It is an extremely reactive element and a strong oxidising agent: Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl. Chlorine Cl Neutron.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Chlorine Cl Neutron Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It is an extremely reactive element and a strong oxidising agent: It has an atomic weight of 35.450 and a mass. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. Only two chlorine isotopes exist. Chlorine is a chemical. Chlorine Cl Neutron.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Chlorine (Cl) YouTube Chlorine Cl Neutron 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. It is an extremely reactive element and a strong oxidising agent: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It has an. Chlorine Cl Neutron.
From www.vedantu.com
Chlorine Learn Definition, Properties and Facts Chlorine Cl Neutron It is an extremely reactive element and a strong oxidising agent: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Naturally occurring. Chlorine Cl Neutron.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Cl Neutron It is an extremely reactive element and a strong oxidising agent: Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Among the. Chlorine Cl Neutron.
From basichemistry.blogspot.com
Basic Chemistry Ions, Cations, and Anions Chlorine Cl Neutron All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Among the elements, it has the highest electron affinity and. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. Chlorine is a chemical element with atomic number 17 which. Chlorine Cl Neutron.
From www.shutterstock.com
Chlorine Cl Periodic Table Element Atomic เวกเตอร์สต็อก (ปลอดค่าลิขสิทธิ์) 2184153385 Chlorine Cl Neutron All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Among the elements, it has the highest electron affinity and. It is an extremely reactive element and a strong oxidising agent: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Only two chlorine isotopes exist. Naturally occurring chlorine consists of 35 cl (mass. Chlorine Cl Neutron.
From circuitdataboattrains.z14.web.core.windows.net
Chlorine Atom Diagram Chlorine Cl Neutron It has an atomic weight of 35.450 and a mass. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Among the elements, it has the highest electron affinity and. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453.. Chlorine Cl Neutron.
From www.alamy.com
Chlorine (Cl). Diagram of the electron configuration of an atom of chlorine (atomic number 17 Chlorine Cl Neutron Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. It has an atomic weight of 35.450 and a mass. It is an extremely reactive element and a strong oxidising agent: Among the elements, it has the highest electron affinity and. Chlorine is the 17th element in the periodic. Chlorine Cl Neutron.
From www.nuclear-power.com
Chlorine Atomic Number Atomic Mass Density of Chlorine Chlorine Cl Neutron Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Only two chlorine isotopes exist. Among the elements, it has the highest electron affinity and. It has an atomic weight of 35.450 and a mass. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. Naturally occurring chlorine consists of 35. Chlorine Cl Neutron.
From www.alamy.com
3d render of atom structure of chlorine isolated over white background Stock Photo Alamy Chlorine Cl Neutron Among the elements, it has the highest electron affinity and. Only two chlorine isotopes exist. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. It has an atomic weight of 35.450 and a. Chlorine Cl Neutron.
From www.slideserve.com
PPT How Many Neutrons? PowerPoint Presentation ID5933204 Chlorine Cl Neutron Only two chlorine isotopes exist. It has an atomic weight of 35.450 and a mass. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. Chlorine is a chemical element. Chlorine Cl Neutron.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chlorine Cl Neutron All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Only two chlorine isotopes exist. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures. Chlorine Cl Neutron.
From slideplayer.com
Illustrating the bonding of Sodium and Chlorine ppt download Chlorine Cl Neutron It has an atomic weight of 35.450 and a mass. Among the elements, it has the highest electron affinity and. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Chlorine is a chemical. Chlorine Cl Neutron.
From elchoroukhost.net
Chlorine Periodic Table Protons Neutrons Electrons Elcho Table Chlorine Cl Neutron Only two chlorine isotopes exist. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23. Chlorine Cl Neutron.
From material-properties.org
Chlorine Protons Neutrons Electrons Electron Configuration Chlorine Cl Neutron Only two chlorine isotopes exist. It is an extremely reactive element and a strong oxidising agent: All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Among the elements, it has the highest electron affinity and. It has an atomic weight of 35.450 and a mass. Naturally occurring chlorine consists of 35. Chlorine Cl Neutron.
From www.sciencephoto.com
Chlorine electron configuration Stock Image C029/5025 Science Photo Library Chlorine Cl Neutron Among the elements, it has the highest electron affinity and. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Only two chlorine isotopes exist. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. It is an extremely reactive element and a strong oxidising agent: It has an atomic weight of 35.450 and. Chlorine Cl Neutron.
From www.shutterstock.com
Atom Chlorine This Diagram Shows Electron Stock Vector 328668782 Shutterstock Chlorine Cl Neutron Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl. Chlorine Cl Neutron.
From elchoroukhost.net
Chlorine Periodic Table Protons Neutrons Electrons Elcho Table Chlorine Cl Neutron Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Sources, facts,. Chlorine Cl Neutron.
From www.pinterest.com
chlorine Atom model project, Electron configuration, Atom model Chlorine Cl Neutron Among the elements, it has the highest electron affinity and. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. All atoms of chlorine (cl) have 17. Chlorine Cl Neutron.
From www.numerade.com
SOLVED 'This image shows............... This image shows points 37 1 Cl 17 Chlorine anion Chlorine Cl Neutron Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. It has an atomic weight of 35.450 and a mass. It. Chlorine Cl Neutron.
From www.slideserve.com
PPT The Halogens PowerPoint Presentation, free download ID6868548 Chlorine Cl Neutron It has an atomic weight of 35.450 and a mass. It is an extremely reactive element and a strong oxidising agent: Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine. Chlorine Cl Neutron.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine Cl Neutron It is an extremely reactive element and a strong oxidising agent: It has an atomic weight of 35.450 and a mass. Only two chlorine isotopes exist. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu),. Chlorine Cl Neutron.
From sciencenotes.org
Chlorine Facts Chlorine Cl Neutron Among the elements, it has the highest electron affinity and. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Chlorine is the. Chlorine Cl Neutron.
From www.dreamstime.com
Diagram Representation of the Element Chlorine Stock Illustration Illustration of energy Chlorine Cl Neutron Among the elements, it has the highest electron affinity and. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Chlorine is the 17th element in the periodic table and has. Chlorine Cl Neutron.
From www.shutterstock.com
3.722 Atom structure of chlorine Görseli, Stok Fotoğraflar ve Vektörler Shutterstock Chlorine Cl Neutron Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine (i.e.. Chlorine Cl Neutron.
From brokeasshome.com
Chlorine Periodic Table Protons Neutrons Electrons Chlorine Cl Neutron Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. It is an extremely reactive element and a strong oxidising agent: Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Only two chlorine isotopes exist. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures. Chlorine Cl Neutron.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Cl Neutron It is an extremely reactive element and a strong oxidising agent: It has an atomic weight of 35.450 and a mass. Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. 53 rows cl by neutron capture is an inevitable consequence of using natural isotope mixtures of chlorine. Chlorine Cl Neutron.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Chlorine Cl Neutron Among the elements, it has the highest electron affinity and. Chlorine is the 17th element in the periodic table and has a symbol of cl and atomic number of 17. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23 neutrons. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Only two chlorine isotopes. Chlorine Cl Neutron.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Cl Neutron Naturally occurring chlorine consists of 35 cl (mass 34.96885 amu) and 37 cl (mass 36.96590 amu), with an average mass of 35.453. Chlorine is a chemical element with atomic number 17 which means there are 17 protons and 17 electrons in the atomic. All atoms of chlorine (cl) have 17 protons, but there are chlorine isotopes having 15 to 23. Chlorine Cl Neutron.