Fe2+ Kmno4 Titration . In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Permanganate ion reduces to a. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture. Potassium permanganate, kmno 4, is a strong oxidizing agent. The redox titration of permanganate ions is commonly used to determine the iron content. Determination of iron content of unknown sample. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or.
from www.numerade.com
The redox titration of permanganate ions is commonly used to determine the iron content. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Determination of iron content of unknown sample. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture. Permanganate ion reduces to a. Potassium permanganate, kmno 4, is a strong oxidizing agent. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution.
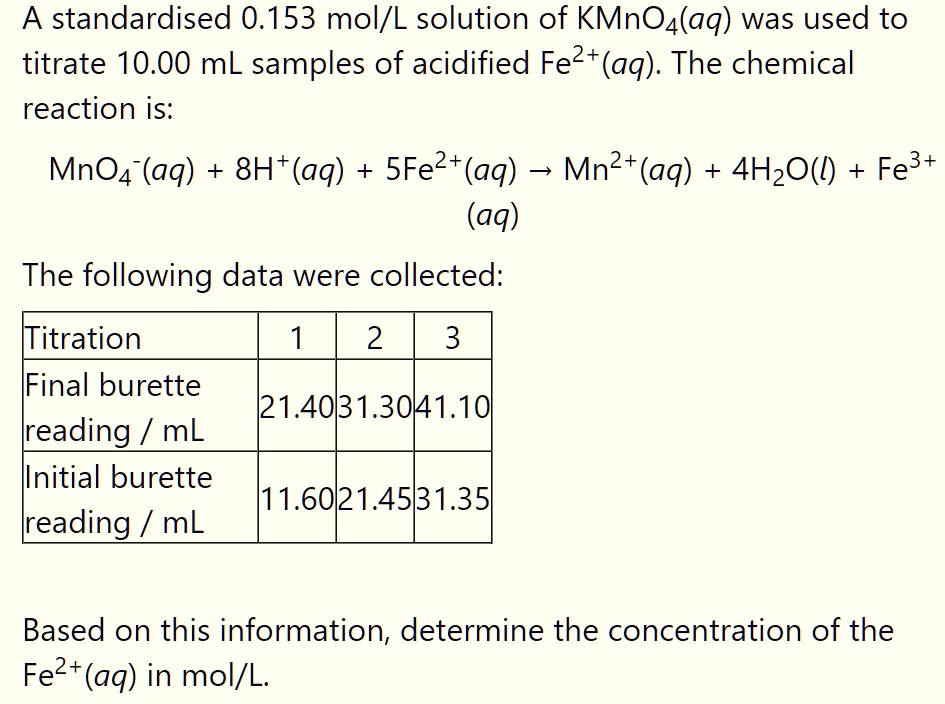
SOLVED A standardized 0.153 mol/L solution of KMnO4(aq) was used to titrate 10.00 mL samples of
Fe2+ Kmno4 Titration The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Determination of iron content of unknown sample. Potassium permanganate, kmno 4, is a strong oxidizing agent. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Permanganate ion reduces to a. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. The redox titration of permanganate ions is commonly used to determine the iron content. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture.
From www.youtube.com
Redox Titration between MnO4 and Fe2+ YouTube Fe2+ Kmno4 Titration Determination of iron content of unknown sample. Potassium permanganate, kmno 4, is a strong oxidizing agent. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. In these redox titrations the manganate (vii) is. Fe2+ Kmno4 Titration.
From www.numerade.com
SOLVED A standardized 0.153 mol/L solution of KMnO4(aq) was used to titrate 10.00 mL samples of Fe2+ Kmno4 Titration Potassium permanganate, kmno 4, is a strong oxidizing agent. Permanganate ion reduces to a. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The redox titration of permanganate ions is commonly used to determine the iron content. In these redox titrations the manganate. Fe2+ Kmno4 Titration.
From www.slideserve.com
PPT The Breathalyzer PowerPoint Presentation, free download ID5480133 Fe2+ Kmno4 Titration Potassium permanganate, kmno 4, is a strong oxidizing agent. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Permanganate ion reduces to a. The redox titration. Fe2+ Kmno4 Titration.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Fe2+ Kmno4 Titration In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The redox titration of permanganate ions is commonly used to determine the iron content. Potassium permanganate, kmno 4, is a strong oxidizing agent. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour,. Fe2+ Kmno4 Titration.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID4545121 Fe2+ Kmno4 Titration In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Potassium permanganate, kmno 4, is a strong oxidizing agent. Permanganate ion reduces to a. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron. Fe2+ Kmno4 Titration.
From www.numerade.com
SOLVED Write the Redox titration report for determination of Fe2+ concentration by Potassium Fe2+ Kmno4 Titration The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Determination of iron content of unknown sample. Permanganate ion reduces to a. Potassium permanganate, kmno 4, is a strong oxidizing agent. In these redox. Fe2+ Kmno4 Titration.
From www.youtube.com
Standardization of KMnO4 Redox Titration KMnO4 Vs Fe2+ Mohrs salt titration YouTube Fe2+ Kmno4 Titration An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture. The kmno4 solution (about 0.02m) is. Fe2+ Kmno4 Titration.
From slideplayer.com
Balance the following MnO4 Mn2+ Fe2+ Fe ppt download Fe2+ Kmno4 Titration In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The redox titration of permanganate ions is commonly used to determine the iron content. Potassium permanganate, kmno 4, is a strong oxidizing agent. In these redox titrations the manganate (vii) is the oxidising agent. Fe2+ Kmno4 Titration.
From www.scribd.com
4 Estimation of Fe2 by KMnO4 PDF Titration Chemistry Fe2+ Kmno4 Titration The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Determination of iron content of unknown sample. Permanganate ion reduces to a. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Potassium permanganate, kmno 4, is a strong oxidizing agent. In these redox. Fe2+ Kmno4 Titration.
From www.youtube.com
How to Balance KMnO4 + Fe = FeO + K2O + MnO2 YouTube Fe2+ Kmno4 Titration Potassium permanganate, kmno 4, is a strong oxidizing agent. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture. The redox titration of permanganate ions is commonly used to determine the iron content. In this. Fe2+ Kmno4 Titration.
From www.numerade.com
SOLVED A titration requires 40.0 mL of 0.10 M KMnO4 to titrate 40.0 mL of Fe2+ solution Fe2+ Kmno4 Titration Permanganate ion reduces to a. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Determination of iron content of unknown sample. In this experiment you will use a standard solution of potassium permanganate. Fe2+ Kmno4 Titration.
From www.youtube.com
FeC2O4+KMnO4+H2SO4 GIVES Fe2(SO4)3+MnSO4+ K2S04+H2O+CO2. Balancing Redox reaction YouTube Fe2+ Kmno4 Titration In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Permanganate ion reduces to a. In these redox titrations the manganate (vii) is the oxidising agent and. Fe2+ Kmno4 Titration.
From docslib.org
Redox Titration Standardization of Kmno4 & the Fe2+ in Iron Pills DocsLib Fe2+ Kmno4 Titration The redox titration of permanganate ions is commonly used to determine the iron content. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. An aqueous solution. Fe2+ Kmno4 Titration.
From www.numerade.com
SOLVED Repeat the titration at least two additional times, recording your data in the data Fe2+ Kmno4 Titration An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture. The redox titration of permanganate ions. Fe2+ Kmno4 Titration.
From www.youtube.com
Titration KMnO4 Vs Mohr Salt in Hindi Full Experiment + Calculations Chemistry Practical Fe2+ Kmno4 Titration An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Determination of iron content of unknown sample. Permanganate ion reduces to a. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Potassium permanganate, kmno. Fe2+ Kmno4 Titration.
From www.numerade.com
SOLVED The Fe2+ content of iron tablets was determined by titration with a freshly standardized Fe2+ Kmno4 Titration Determination of iron content of unknown sample. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. The redox titration of permanganate ions is commonly used to determine the iron content. In these redox titrations the manganate (vii) is the oxidising agent and is. Fe2+ Kmno4 Titration.
From www.numerade.com
SOLVEDRedox Titrationi Acidic Medium Puch Slider Upto Add Volume of KMnO Tetal Volume of KMno4 Fe2+ Kmno4 Titration Determination of iron content of unknown sample. Potassium permanganate, kmno 4, is a strong oxidizing agent. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture. In this experiment you will use a standard solution. Fe2+ Kmno4 Titration.
From www.numerade.com
A KMnO4 solution is to be standardized by titration against As2O3. A 0.1078 g sample of As2O3 Fe2+ Kmno4 Titration Potassium permanganate, kmno 4, is a strong oxidizing agent. Permanganate ion reduces to a. The redox titration of permanganate ions is commonly used to determine the iron content. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. In these redox titrations the manganate. Fe2+ Kmno4 Titration.
From www.youtube.com
Redox Titration of FeSO4 with KMnO4 and Calculation of Equivalance point Potential YouTube Fe2+ Kmno4 Titration An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Potassium permanganate, kmno 4, is a strong oxidizing agent. The redox titration of permanganate ions is commonly used to determine the iron content. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq). Fe2+ Kmno4 Titration.
From www.numerade.com
SOLVED A redox titration using a molar solution of potassium permanganate (KMnO4) against a Fe2+ Kmno4 Titration The redox titration of permanganate ions is commonly used to determine the iron content. Potassium permanganate, kmno 4, is a strong oxidizing agent. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of. Fe2+ Kmno4 Titration.
From www.chegg.com
Solved 4. In this experiment; as you perform redox titration Fe2+ Kmno4 Titration An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Permanganate ion reduces to a. The redox titration of permanganate ions is commonly used to. Fe2+ Kmno4 Titration.
From www.youtube.com
titration of kmno4 with mohr's salt class 12 determine the concentration of KMnO4 using mohr Fe2+ Kmno4 Titration Potassium permanganate, kmno 4, is a strong oxidizing agent. Permanganate ion reduces to a. The redox titration of permanganate ions is commonly used to determine the iron content. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and. Fe2+ Kmno4 Titration.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记6.1.9 Redox Titration Calculations翰林国际教育 Fe2+ Kmno4 Titration Permanganate ion reduces to a. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Potassium permanganate, kmno 4, is a strong oxidizing agent. In. Fe2+ Kmno4 Titration.
From slideplayer.com
Volumetric Analysis OxidationReduction ppt download Fe2+ Kmno4 Titration In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture. Permanganate ion reduces to a. The redox titration of permanganate ions is commonly used to determine the iron content. An aqueous solution of potassium permanganate,. Fe2+ Kmno4 Titration.
From www.youtube.com
Titration Of Kmno4 Vs Oxalic Acid The Ultimate Guide Titration KMnO4 vs oxalic acid Fe2+ Kmno4 Titration The redox titration of permanganate ions is commonly used to determine the iron content. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Determination of iron content of unknown sample. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if. Fe2+ Kmno4 Titration.
From www.youtube.com
Determination of Concentration of KMnO4 Soution using Ferrous Ammonium Sulphate MeitY OLabs Fe2+ Kmno4 Titration Determination of iron content of unknown sample. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as fe 2+) in an unknown solution. Permanganate ion reduces to a. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. The redox titration. Fe2+ Kmno4 Titration.
From www.researchgate.net
Effect of solution pH on phosphate removal using Fe 2 þ KMnO 4... Download Scientific Diagram Fe2+ Kmno4 Titration The redox titration of permanganate ions is commonly used to determine the iron content. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture. In this experiment you will use a standard solution of potassium. Fe2+ Kmno4 Titration.
From www.numerade.com
SOLVED Write the Redox titration report for determination of Fe2+ concentration by Potassium Fe2+ Kmno4 Titration Potassium permanganate, kmno 4, is a strong oxidizing agent. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate. Fe2+ Kmno4 Titration.
From chemistrypubs.com
Titration of Oxalic Acid with KMnO4 Chemistrupubs Fe2+ Kmno4 Titration An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. The redox titration of permanganate ions is commonly used to determine the iron content. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised. Fe2+ Kmno4 Titration.
From www.slideserve.com
PPT Redox Titrations PowerPoint Presentation, free download ID2952040 Fe2+ Kmno4 Titration The redox titration of permanganate ions is commonly used to determine the iron content. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture. In this experiment you will use a standard solution of potassium. Fe2+ Kmno4 Titration.
From www.slideserve.com
PPT REDOX TITRATION PowerPoint Presentation, free download ID431911 Fe2+ Kmno4 Titration In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+ (aq) the iron is the reducing agent and is oxidised to fe 2+ (aq) and the reaction mixture. The redox titration of permanganate ions is commonly used to determine the iron content. Determination of iron content of unknown sample. Potassium permanganate, kmno 4,. Fe2+ Kmno4 Titration.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Fe2+ Kmno4 Titration An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Determination of iron content of unknown sample. The redox titration of permanganate ions is commonly used to determine the iron content. In this experiment you will use a standard solution of potassium permanganate (kmno 4) to determine the of iron (as. Fe2+ Kmno4 Titration.
From www.numerade.com
SOLVED The KMnO4 solution is first standardized by titration using Mohr's salt; ferrous Fe2+ Kmno4 Titration Determination of iron content of unknown sample. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. In these redox titrations the manganate (vii) is the oxidising agent and is reduced to mn 2+. Fe2+ Kmno4 Titration.
From www.numerade.com
SOLVED In the titration of ammonium iron (II) sulfate hexahydrate with a standard solution of Fe2+ Kmno4 Titration Permanganate ion reduces to a. The redox titration of permanganate ions is commonly used to determine the iron content. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Potassium permanganate, kmno 4, is a strong oxidizing agent. In these redox titrations the manganate (vii) is the oxidising agent and is. Fe2+ Kmno4 Titration.
From www.studypool.com
SOLUTION chemistry practical layout to find strength of given Mohr salt solution Fe2+ Kmno4 Titration Potassium permanganate, kmno 4, is a strong oxidizing agent. An aqueous solution of potassium permanganate, $\ce{kmno4}$ is of purple colour, regardless if the solution is neutral, acidic, or. Permanganate ion reduces to a. The kmno4 solution (about 0.02m) is first standardized by titration using mohr’s salt, ferrous ammonium sulfate hexahydrate,. Determination of iron content of unknown sample. The redox titration. Fe2+ Kmno4 Titration.