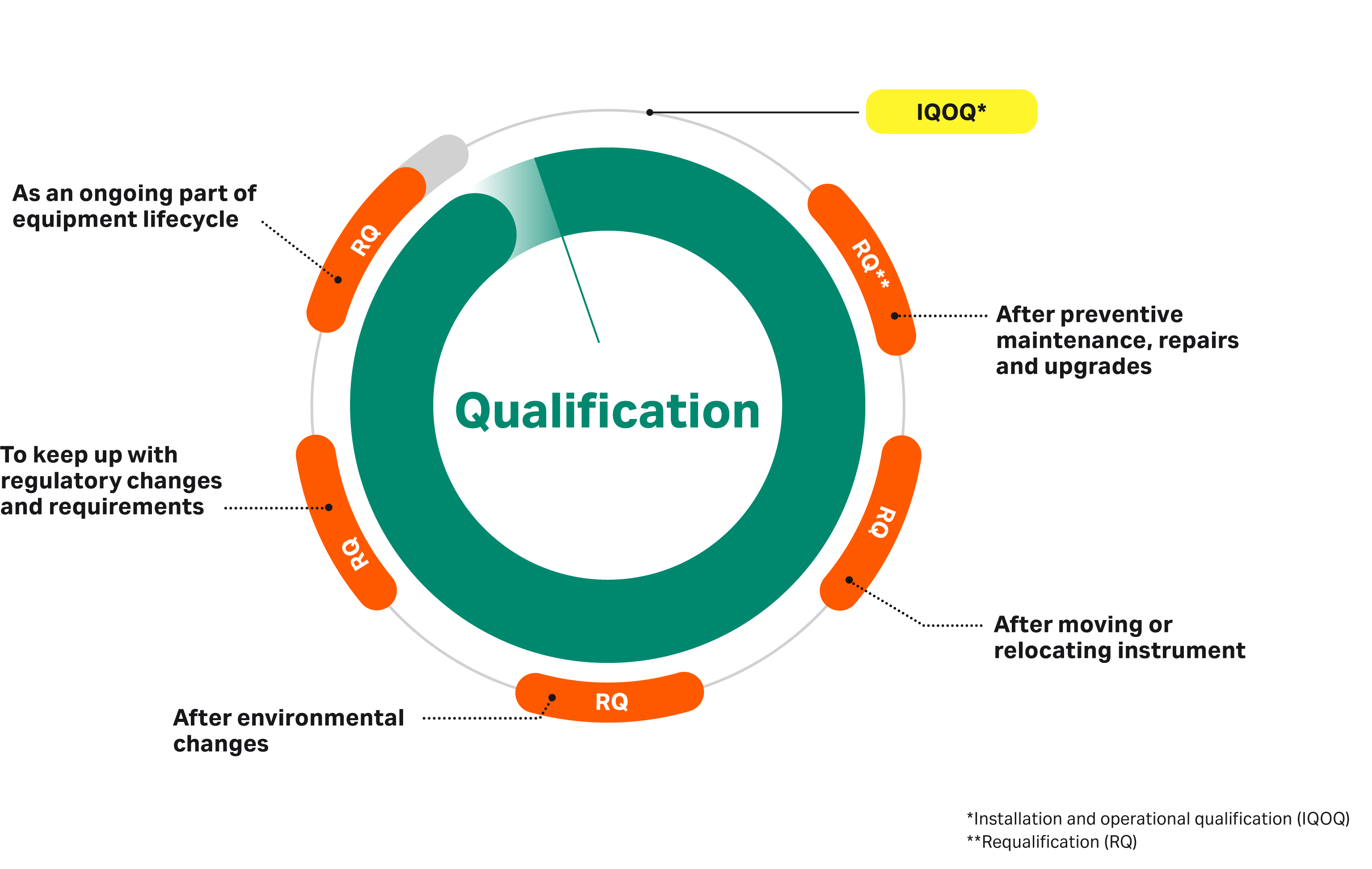
Equipment Performance Qualification . Equipment is verified to perform within the acceptable and specified range under normal operating conditions. pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. User requirements are evaluated to ensure that the requirements are met. what is iq, oq, pq? qualifications are the actions of proving through documented evidence that any premise, system, and items of equipment work correctly. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. What are they and why are they important? pq stands for performance qualification. It is the final step in verifying, validating, or qualifying equipment. These are the abbreviations we use in the medical device industry. iq, oq, and pq: Explore the profound significance of iq, oq, and pq in regulated industries. performance qualification is a part of equipment validation process and there are a number of reasons why.
from www.cytivalifesciences.com
Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. qualifications are the actions of proving through documented evidence that any premise, system, and items of equipment work correctly. performance qualification is a part of equipment validation process and there are a number of reasons why. pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. iq, oq, and pq: Explore the profound significance of iq, oq, and pq in regulated industries. User requirements are evaluated to ensure that the requirements are met. what is iq, oq, pq? What are they and why are they important? It is the final step in verifying, validating, or qualifying equipment.
Stay compliant instrument qualification for cGMP Cytiva
Equipment Performance Qualification It is the final step in verifying, validating, or qualifying equipment. performance qualification is a part of equipment validation process and there are a number of reasons why. Explore the profound significance of iq, oq, and pq in regulated industries. What are they and why are they important? It is the final step in verifying, validating, or qualifying equipment. These are the abbreviations we use in the medical device industry. pq stands for performance qualification. pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. User requirements are evaluated to ensure that the requirements are met. Equipment is verified to perform within the acceptable and specified range under normal operating conditions. qualifications are the actions of proving through documented evidence that any premise, system, and items of equipment work correctly. what is iq, oq, pq? iq, oq, and pq: Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation ID307459 Equipment Performance Qualification Equipment is verified to perform within the acceptable and specified range under normal operating conditions. It is the final step in verifying, validating, or qualifying equipment. iq, oq, and pq: pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq). Equipment Performance Qualification.
From pharmaguidances.com
Equipment Qualification Pharmaceutical Guidance Equipment Performance Qualification It is the final step in verifying, validating, or qualifying equipment. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. performance qualification is a part of equipment validation process and there are a number of reasons why. Equipment is verified to perform within the acceptable and specified range under normal operating conditions. User requirements are. Equipment Performance Qualification.
From www.olanabconsults.com
The Four Stages of Equipment Qualification Explained Equipment Performance Qualification Equipment is verified to perform within the acceptable and specified range under normal operating conditions. pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. performance qualification is a part of equipment validation process and there are a number of reasons why. It is the final step in verifying, validating,. Equipment Performance Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Equipment Performance Qualification User requirements are evaluated to ensure that the requirements are met. pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. It is the final step in verifying, validating, or qualifying equipment. what is iq, oq, pq? pq stands for performance qualification. What are they and why are they. Equipment Performance Qualification.
From www.slideserve.com
PPT 3) Safety Considerations PowerPoint Presentation, free download Equipment Performance Qualification User requirements are evaluated to ensure that the requirements are met. what is iq, oq, pq? performance qualification is a part of equipment validation process and there are a number of reasons why. These are the abbreviations we use in the medical device industry. It is the final step in verifying, validating, or qualifying equipment. pq stands. Equipment Performance Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Equipment Performance Qualification Explore the profound significance of iq, oq, and pq in regulated industries. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. performance qualification is a part of equipment validation process and there are a number of reasons why. pq stands for performance qualification. Equipment is verified to perform within the acceptable and specified range. Equipment Performance Qualification.
From www.slideserve.com
PPT Statistical Tools, Performance Verification PowerPoint Equipment Performance Qualification These are the abbreviations we use in the medical device industry. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. It is the final step in verifying, validating, or qualifying equipment. User requirements are evaluated to ensure that the requirements are met. iq, oq, and pq: performance qualification is a part of equipment validation. Equipment Performance Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Equipment Performance Qualification Explore the profound significance of iq, oq, and pq in regulated industries. These are the abbreviations we use in the medical device industry. performance qualification is a part of equipment validation process and there are a number of reasons why. What are they and why are they important? what is iq, oq, pq? pq (performance qualification) confirms. Equipment Performance Qualification.
From www.scribd.com
Coating Equipment Performance Qualification PDF Tablet (Pharmacy Equipment Performance Qualification User requirements are evaluated to ensure that the requirements are met. what is iq, oq, pq? iq, oq, and pq: Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. qualifications are the actions of proving through documented evidence that any premise, system, and items of equipment work correctly. performance qualification is a. Equipment Performance Qualification.
From www.slideshare.net
Equipment qualification Equipment Performance Qualification Explore the profound significance of iq, oq, and pq in regulated industries. iq, oq, and pq: Equipment is verified to perform within the acceptable and specified range under normal operating conditions. These are the abbreviations we use in the medical device industry. qualifications are the actions of proving through documented evidence that any premise, system, and items of. Equipment Performance Qualification.
From mpl.loesungsfabrik.de
What is qualification? Equipment Performance Qualification iq, oq, and pq: Equipment is verified to perform within the acceptable and specified range under normal operating conditions. It is the final step in verifying, validating, or qualifying equipment. pq stands for performance qualification. Explore the profound significance of iq, oq, and pq in regulated industries. User requirements are evaluated to ensure that the requirements are met.. Equipment Performance Qualification.
From www.slideshare.net
1 5 equipmentqualification Equipment Performance Qualification It is the final step in verifying, validating, or qualifying equipment. qualifications are the actions of proving through documented evidence that any premise, system, and items of equipment work correctly. What are they and why are they important? pq stands for performance qualification. Explore the profound significance of iq, oq, and pq in regulated industries. Us pharmacopeia (usp). Equipment Performance Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Equipment Performance Qualification what is iq, oq, pq? Equipment is verified to perform within the acceptable and specified range under normal operating conditions. What are they and why are they important? These are the abbreviations we use in the medical device industry. pq stands for performance qualification. User requirements are evaluated to ensure that the requirements are met. It is the. Equipment Performance Qualification.
From www.pharmaqualification.com
Performance Qualification Equipment Performance Qualification iq, oq, and pq: Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. These are the abbreviations we use in the medical device industry. It is the final step in verifying, validating, or qualifying equipment. User requirements are evaluated to ensure that the requirements are met. Equipment is verified to perform within the acceptable and. Equipment Performance Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Equipment Performance Qualification Explore the profound significance of iq, oq, and pq in regulated industries. qualifications are the actions of proving through documented evidence that any premise, system, and items of equipment work correctly. iq, oq, and pq: pq stands for performance qualification. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. what is iq,. Equipment Performance Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Equipment Performance Qualification Explore the profound significance of iq, oq, and pq in regulated industries. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. what is iq, oq, pq? iq, oq, and pq: User requirements are evaluated to ensure that the requirements are met. pq stands for performance qualification. It is the final step in verifying,. Equipment Performance Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Equipment Performance Qualification pq stands for performance qualification. pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. What are they and why are they important? Explore the profound significance of iq, oq, and pq in regulated industries. It is the final step in verifying, validating, or qualifying equipment. performance qualification is. Equipment Performance Qualification.
From studylib.net
Equipment Qualification Equipment Performance Qualification pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. What are they and why are they important? Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. Explore the profound significance of iq, oq, and pq in regulated industries. It is the final step in verifying, validating,. Equipment Performance Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Equipment Performance Qualification pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. These are the abbreviations we use in the medical device industry. pq stands for performance qualification. performance qualification is a part of equipment validation process and there are a number of reasons why. Us pharmacopeia (usp) general chapter on. Equipment Performance Qualification.
From pharmaanalytic.com
Equipment Qualification Equipment Performance Qualification These are the abbreviations we use in the medical device industry. Explore the profound significance of iq, oq, and pq in regulated industries. Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. It is the final step in verifying, validating, or qualifying equipment. pq (performance qualification) confirms that once installed and operating, the equipment consistently. Equipment Performance Qualification.
From www.scribd.com
The Importance of Installation Qualification, Operational Qualification Equipment Performance Qualification iq, oq, and pq: pq stands for performance qualification. Equipment is verified to perform within the acceptable and specified range under normal operating conditions. User requirements are evaluated to ensure that the requirements are met. what is iq, oq, pq? It is the final step in verifying, validating, or qualifying equipment. pq (performance qualification) confirms that. Equipment Performance Qualification.
From pharmaguidances.com
Equipment Qualification Pharmaceutical Guidance Equipment Performance Qualification Equipment is verified to perform within the acceptable and specified range under normal operating conditions. User requirements are evaluated to ensure that the requirements are met. Explore the profound significance of iq, oq, and pq in regulated industries. performance qualification is a part of equipment validation process and there are a number of reasons why. What are they and. Equipment Performance Qualification.
From www.slidegeeks.com
Four Stages Equipment Qualification Framework Ppt PowerPoint Equipment Performance Qualification Equipment is verified to perform within the acceptable and specified range under normal operating conditions. Explore the profound significance of iq, oq, and pq in regulated industries. What are they and why are they important? Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. performance qualification is a part of equipment validation process and there. Equipment Performance Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Equipment Performance Qualification It is the final step in verifying, validating, or qualifying equipment. Equipment is verified to perform within the acceptable and specified range under normal operating conditions. pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. performance qualification is a part of equipment validation process and there are a number. Equipment Performance Qualification.
From www.cytivalifesciences.com
Stay compliant instrument qualification for cGMP Cytiva Equipment Performance Qualification iq, oq, and pq: Equipment is verified to perform within the acceptable and specified range under normal operating conditions. qualifications are the actions of proving through documented evidence that any premise, system, and items of equipment work correctly. What are they and why are they important? Explore the profound significance of iq, oq, and pq in regulated industries.. Equipment Performance Qualification.
From www.youtube.com
Equipment Qualification YouTube Equipment Performance Qualification pq stands for performance qualification. Equipment is verified to perform within the acceptable and specified range under normal operating conditions. Explore the profound significance of iq, oq, and pq in regulated industries. iq, oq, and pq: what is iq, oq, pq? These are the abbreviations we use in the medical device industry. What are they and why. Equipment Performance Qualification.
From pdfslide.net
(PDF) General Laboratory Equipment Performance Qualification Equipment Performance Qualification what is iq, oq, pq? qualifications are the actions of proving through documented evidence that any premise, system, and items of equipment work correctly. It is the final step in verifying, validating, or qualifying equipment. Equipment is verified to perform within the acceptable and specified range under normal operating conditions. Us pharmacopeia (usp) general chapter on analytical instrument. Equipment Performance Qualification.
From www.youtube.com
Performance Qualification (PQ) Equipment Qualification What is Equipment Performance Qualification Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. pq stands for performance qualification. iq, oq, and pq: These are the abbreviations we use in the medical device industry. What are they and why are they important? User requirements are evaluated to ensure that the requirements are met. what is iq, oq, pq?. Equipment Performance Qualification.
From cektbehb.blob.core.windows.net
What Is A Equipment Performance at Pauline Butler blog Equipment Performance Qualification iq, oq, and pq: Equipment is verified to perform within the acceptable and specified range under normal operating conditions. pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. What are they and why are they important? qualifications are the actions of proving through documented evidence that any premise,. Equipment Performance Qualification.
From www.slideserve.com
PPT VALIDATION METHODOLOGY PowerPoint Presentation, free download Equipment Performance Qualification What are they and why are they important? qualifications are the actions of proving through documented evidence that any premise, system, and items of equipment work correctly. pq stands for performance qualification. User requirements are evaluated to ensure that the requirements are met. what is iq, oq, pq? performance qualification is a part of equipment validation. Equipment Performance Qualification.
From gmptemplates.com
E038001 EQUIPMENT QUALIFICATION PROCEDURE GMP Templates Equipment Performance Qualification These are the abbreviations we use in the medical device industry. what is iq, oq, pq? It is the final step in verifying, validating, or qualifying equipment. pq stands for performance qualification. Equipment is verified to perform within the acceptable and specified range under normal operating conditions. iq, oq, and pq: qualifications are the actions of. Equipment Performance Qualification.
From www.slideserve.com
PPT Validation of capsule filling machine PowerPoint Presentation Equipment Performance Qualification It is the final step in verifying, validating, or qualifying equipment. pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. What are they and why are they important? Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. qualifications are the actions of proving through documented. Equipment Performance Qualification.
From www.slideserve.com
PPT EQUIPMENT AND ITS QUALIFICATION PowerPoint Presentation, free Equipment Performance Qualification qualifications are the actions of proving through documented evidence that any premise, system, and items of equipment work correctly. User requirements are evaluated to ensure that the requirements are met. pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product meeting its predetermined. performance qualification is a part of equipment validation process. Equipment Performance Qualification.
From www.vrogue.co
Equipment Qualification Procedure And Protocol Guidel vrogue.co Equipment Performance Qualification what is iq, oq, pq? Explore the profound significance of iq, oq, and pq in regulated industries. Equipment is verified to perform within the acceptable and specified range under normal operating conditions. User requirements are evaluated to ensure that the requirements are met. pq (performance qualification) confirms that once installed and operating, the equipment consistently produces a product. Equipment Performance Qualification.
From www.researchgate.net
(PDF) Best Practices in Freeze Dryer Equipment Performance Equipment Performance Qualification What are they and why are they important? Us pharmacopeia (usp) general chapter on analytical instrument qualification (aiq) was first implemented. User requirements are evaluated to ensure that the requirements are met. qualifications are the actions of proving through documented evidence that any premise, system, and items of equipment work correctly. These are the abbreviations we use in the. Equipment Performance Qualification.