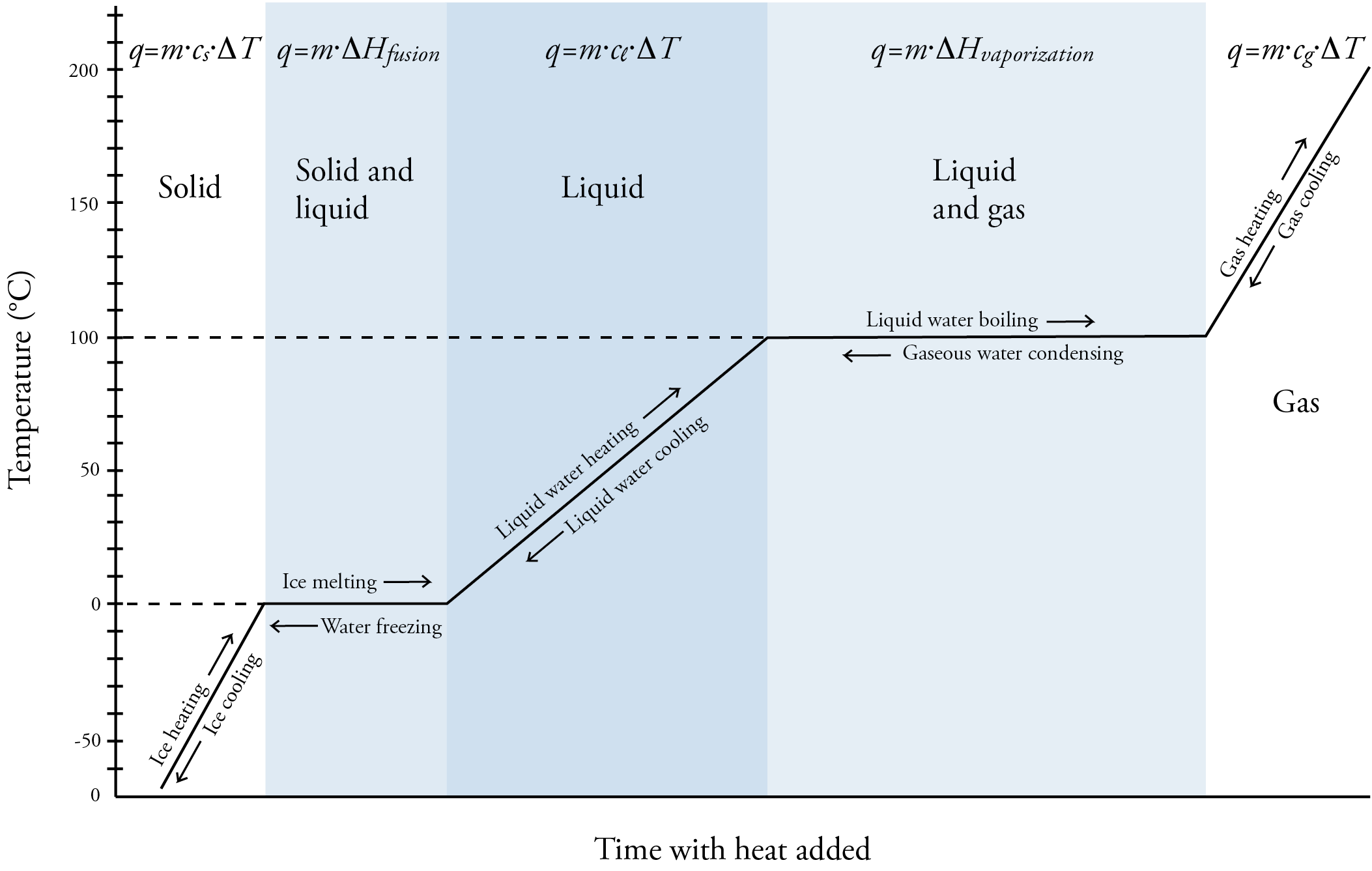
The Heating Curve Of Water From Ice To Vapor Is . A heating curve for water. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. Δ h v a p \delta h_ {vap}. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. liquid water becomes water vapor or steam when it enters the gaseous phase. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. Use the heat of vaporization (. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. The water could then be cooled to 0°c, at which point.
from studyschoolford.z21.web.core.windows.net
A heating curve for water. The water could then be cooled to 0°c, at which point. Use the heat of vaporization (. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. Δ h v a p \delta h_ {vap}. liquid water becomes water vapor or steam when it enters the gaseous phase. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water.
Heating Curve Of Water Diagram
The Heating Curve Of Water From Ice To Vapor Is The water could then be cooled to 0°c, at which point. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. A heating curve for water. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. The water could then be cooled to 0°c, at which point. liquid water becomes water vapor or steam when it enters the gaseous phase. Use the heat of vaporization (. Δ h v a p \delta h_ {vap}.
From chem-net.blogspot.com
Phase Changes Energy Changes Heating Curves Chemistry Net The Heating Curve Of Water From Ice To Vapor Is as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. The water could then be cooled to 0°c, at which point. Δ h v a p \delta h_ {vap}. Use the heat of vaporization (. our water heating calculator can help you determine both the amount of heat required to raise. The Heating Curve Of Water From Ice To Vapor Is.
From www.researchgate.net
Heating curve for water. Download Scientific Diagram The Heating Curve Of Water From Ice To Vapor Is a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. A heating curve for water. as heat is added to water, the temperature increases which. The Heating Curve Of Water From Ice To Vapor Is.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Explained The Heating Curve Of Water From Ice To Vapor Is The water could then be cooled to 0°c, at which point. Δ h v a p \delta h_ {vap}. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. as. The Heating Curve Of Water From Ice To Vapor Is.
From scienceisntscary.wordpress.com
Image The Heating Curve Of Water From Ice To Vapor Is Δ h v a p \delta h_ {vap}. Use the heat of vaporization (. liquid water becomes water vapor or steam when it enters the gaseous phase. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. A heating curve for water. as heat is added to water,. The Heating Curve Of Water From Ice To Vapor Is.
From kittyx-tomow.blogspot.com
Heating Curve Of Water Heating Curve Of Water Heating curve basics The Heating Curve Of Water From Ice To Vapor Is steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. The water could then be cooled to 0°c, at which point. our water heating calculator can help you determine both the. The Heating Curve Of Water From Ice To Vapor Is.
From cevwmhpq.blob.core.windows.net
Heating Curve Of Water Examples at Levi Bowen blog The Heating Curve Of Water From Ice To Vapor Is steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. Δ h v a p \delta h_ {vap}. as heat is added to water, the. The Heating Curve Of Water From Ice To Vapor Is.
From brainly.in
To study the effect of heat on ice by using a graph Brainly.in The Heating Curve Of Water From Ice To Vapor Is A heating curve for water. Use the heat of vaporization (. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. steam above 100°c could be. The Heating Curve Of Water From Ice To Vapor Is.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Explained The Heating Curve Of Water From Ice To Vapor Is a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. Use the heat of vaporization (. steam above 100°c could be steadily cooled down to 100°c, at. The Heating Curve Of Water From Ice To Vapor Is.
From ch302.cm.utexas.edu
heating curve The Heating Curve Of Water From Ice To Vapor Is The water could then be cooled to 0°c, at which point. liquid water becomes water vapor or steam when it enters the gaseous phase. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. steam above 100°c could be steadily cooled down to 100°c,. The Heating Curve Of Water From Ice To Vapor Is.
From www.gauthmath.com
The figure below shows the heating curve of water with four labeled The Heating Curve Of Water From Ice To Vapor Is The water could then be cooled to 0°c, at which point. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. Δ h v a p \delta. The Heating Curve Of Water From Ice To Vapor Is.
From studyschoolford.z21.web.core.windows.net
Heating Curve Of Water Explained The Heating Curve Of Water From Ice To Vapor Is A heating curve for water. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. liquid water becomes water vapor or steam when it enters the gaseous phase. Use the heat of vaporization (. our water heating calculator can help you determine both the amount of heat required. The Heating Curve Of Water From Ice To Vapor Is.
From www.pinterest.com
Heating Curve of Water Resource classroom, Intermolecular force The Heating Curve Of Water From Ice To Vapor Is a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. our water heating calculator can help you determine both the amount of heat required to raise. The Heating Curve Of Water From Ice To Vapor Is.
From lessonschoolwinchell.z21.web.core.windows.net
Heating Curve Of Water Chart The Heating Curve Of Water From Ice To Vapor Is A heating curve for water. The water could then be cooled to 0°c, at which point. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. Use the heat of vaporization (. Δ h v a p \delta h_ {vap}.. The Heating Curve Of Water From Ice To Vapor Is.
From studylane55.z19.web.core.windows.net
Heat Curve Of Water The Heating Curve Of Water From Ice To Vapor Is The water could then be cooled to 0°c, at which point. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. A heating curve for water. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. Δ h. The Heating Curve Of Water From Ice To Vapor Is.
From guidelibephemerist.z14.web.core.windows.net
Phase Diagrams Understanding The Basics The Heating Curve Of Water From Ice To Vapor Is Δ h v a p \delta h_ {vap}. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. our water heating calculator can help you determine both. The Heating Curve Of Water From Ice To Vapor Is.
From chem.libretexts.org
11.7 Heating Curve for Water Chemistry LibreTexts The Heating Curve Of Water From Ice To Vapor Is Use the heat of vaporization (. Δ h v a p \delta h_ {vap}. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h. The Heating Curve Of Water From Ice To Vapor Is.
From learningschoolgraciauwb.z4.web.core.windows.net
Heating Curve Of Water Pdf The Heating Curve Of Water From Ice To Vapor Is liquid water becomes water vapor or steam when it enters the gaseous phase. Δ h v a p \delta h_ {vap}. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of. The Heating Curve Of Water From Ice To Vapor Is.
From studylib.net
Heating Curve of Water The Heating Curve Of Water From Ice To Vapor Is Use the heat of vaporization (. liquid water becomes water vapor or steam when it enters the gaseous phase. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. The water could then be cooled to 0°c, at which point. our water heating calculator can help you determine both the. The Heating Curve Of Water From Ice To Vapor Is.
From worksheetfullpemmican.z22.web.core.windows.net
Heating Curve Of Water Answers The Heating Curve Of Water From Ice To Vapor Is Δ h v a p \delta h_ {vap}. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. liquid. The Heating Curve Of Water From Ice To Vapor Is.
From worksheetdbyrent.z19.web.core.windows.net
Heat Curve Of Water The Heating Curve Of Water From Ice To Vapor Is Δ h v a p \delta h_ {vap}. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. liquid water becomes water vapor or steam when it enters the gaseous phase. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. . The Heating Curve Of Water From Ice To Vapor Is.
From www.slideserve.com
PPT Heating Curve for Water PowerPoint Presentation, free download The Heating Curve Of Water From Ice To Vapor Is This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. liquid water becomes water vapor or steam when it enters the gaseous phase. A heating curve for water. The water could then be cooled to 0°c, at which point. Use the heat of vaporization (. a) a heating curve. The Heating Curve Of Water From Ice To Vapor Is.
From shaunmwilliams.com
Chapter 10 Presentation The Heating Curve Of Water From Ice To Vapor Is This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. The water could then be cooled to 0°c, at which point.. The Heating Curve Of Water From Ice To Vapor Is.
From www.slideserve.com
PPT Chapter 16 Reaction Energy PowerPoint Presentation, free download The Heating Curve Of Water From Ice To Vapor Is Use the heat of vaporization (. liquid water becomes water vapor or steam when it enters the gaseous phase. The water could then be cooled to 0°c, at which point. A heating curve for water. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. as heat is added. The Heating Curve Of Water From Ice To Vapor Is.
From www.slideserve.com
PPT Heating Curves and Thermodynamics PowerPoint Presentation, free The Heating Curve Of Water From Ice To Vapor Is A heating curve for water. Δ h v a p \delta h_ {vap}. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. as heat is added to water, the temperature increases which increases the kinetic energy of the. The Heating Curve Of Water From Ice To Vapor Is.
From www.chegg.com
Solved 12. This diagram shows a heating curve for ice The Heating Curve Of Water From Ice To Vapor Is Δ h v a p \delta h_ {vap}. A heating curve for water. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. liquid water becomes water vapor or steam when. The Heating Curve Of Water From Ice To Vapor Is.
From worksheetfullpemmican.z22.web.core.windows.net
Heating And Cooling Curves Explained The Heating Curve Of Water From Ice To Vapor Is steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. The water could then be cooled to 0°c, at which point. Use the heat of vaporization (. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2. The Heating Curve Of Water From Ice To Vapor Is.
From deaiszkoeco.blob.core.windows.net
Heating Curve Of Water Experiment Theory at Laura Short blog The Heating Curve Of Water From Ice To Vapor Is steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. This plot of temperature shows what happens to a 75 g sample of ice initially at. The Heating Curve Of Water From Ice To Vapor Is.
From worksheetfullpemmican.z22.web.core.windows.net
Heating Curve Of Water Explained The Heating Curve Of Water From Ice To Vapor Is A heating curve for water. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. steam above 100°c could be steadily cooled down to 100°c, at which. The Heating Curve Of Water From Ice To Vapor Is.
From cevwmhpq.blob.core.windows.net
Heating Curve Of Water Examples at Levi Bowen blog The Heating Curve Of Water From Ice To Vapor Is as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. steam above 100°c could be steadily cooled down to 100°c, at which point it would condense to liquid water. Δ h v a p \delta h_ {vap}. liquid water becomes water vapor or steam when it enters the gaseous phase.. The Heating Curve Of Water From Ice To Vapor Is.
From www.youtube.com
Heating Curve and Cooling Curve of Water Enthalpy of Fusion The Heating Curve Of Water From Ice To Vapor Is Use the heat of vaporization (. liquid water becomes water vapor or steam when it enters the gaseous phase. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm and. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. a) a. The Heating Curve Of Water From Ice To Vapor Is.
From www.scribd.com
The Heating Curve of Water PDF Ice Latent Heat The Heating Curve Of Water From Ice To Vapor Is a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. Use the heat of vaporization (. our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take.. The Heating Curve Of Water From Ice To Vapor Is.
From slideplayer.com
ATMOSPHERIC ENERGY. ppt download The Heating Curve Of Water From Ice To Vapor Is a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. liquid water becomes water vapor or steam when it enters the gaseous phase. Use the heat of vaporization (. This plot of temperature shows what happens to a 75 g sample of ice initially at. The Heating Curve Of Water From Ice To Vapor Is.
From www.gauthmath.com
The figure below shows the heating curve of water with four labeled The Heating Curve Of Water From Ice To Vapor Is our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. The water could then be cooled to 0°c, at which point. Δ h v a p \delta h_ {vap}. Use the heat of vaporization (. a) a heating curve. The Heating Curve Of Water From Ice To Vapor Is.
From quizzschoolhernandez.z19.web.core.windows.net
Heating Curve Of Water Explained The Heating Curve Of Water From Ice To Vapor Is our water heating calculator can help you determine both the amount of heat required to raise the temperature of some h 2 o and the time it will take. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. The water could then be cooled to 0°c, at which point. . The Heating Curve Of Water From Ice To Vapor Is.
From studyschoolford.z21.web.core.windows.net
Heating Curve Of Water Diagram The Heating Curve Of Water From Ice To Vapor Is a) a heating curve for water depicts changes in temperature that result as the substance absorbs increasing amounts of heat at 1 atm. as heat is added to water, the temperature increases which increases the kinetic energy of the molecules. This plot of temperature shows what happens to a 75 g sample of ice initially at 1 atm. The Heating Curve Of Water From Ice To Vapor Is.