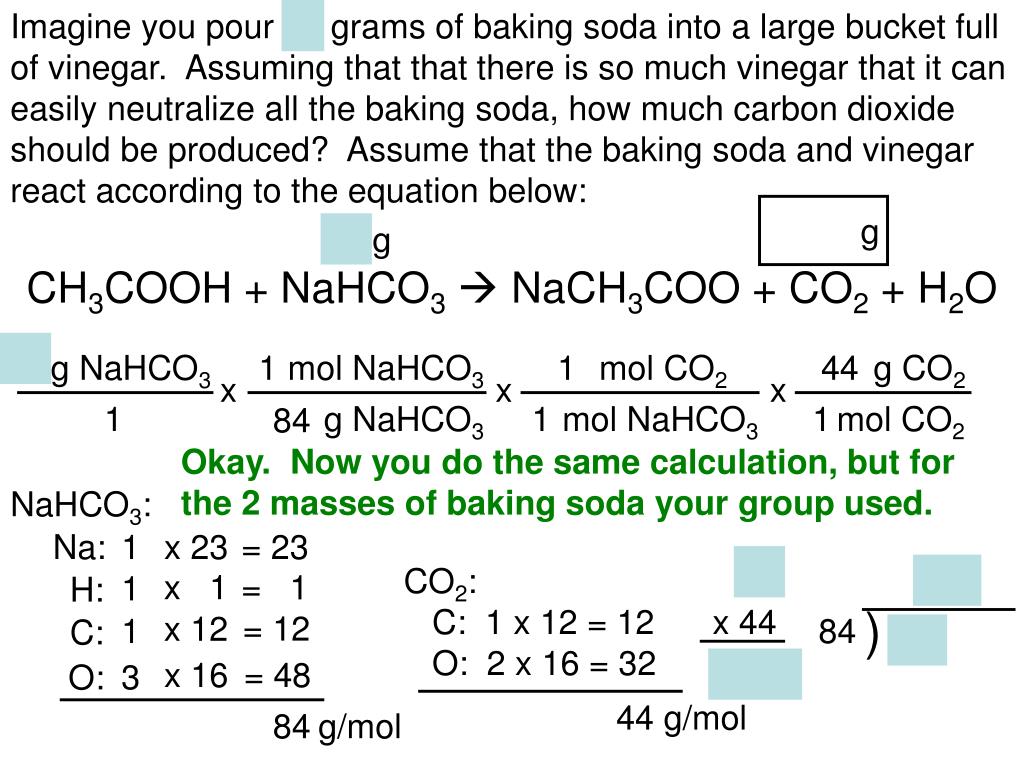Baking Soda And Vinegar Lab Answers . O put the balloon over the mouth of. The balanced chemical equation is: what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: O put in 30 ml of vinegar in the bottle. a gas that was not created, but came out of the baking soda particles during the reaction with vinegar (co2) Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. o put a heaping spoonful of baking soda in the balloon. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar.
from www.slideserve.com
O put the balloon over the mouth of. the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: The balanced chemical equation is: O put in 30 ml of vinegar in the bottle. o put a heaping spoonful of baking soda in the balloon. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. a gas that was not created, but came out of the baking soda particles during the reaction with vinegar (co2)
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint
Baking Soda And Vinegar Lab Answers The balanced chemical equation is: The balanced chemical equation is: Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. O put in 30 ml of vinegar in the bottle. o put a heaping spoonful of baking soda in the balloon. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. a gas that was not created, but came out of the baking soda particles during the reaction with vinegar (co2) O put the balloon over the mouth of.
From www.studocu.com
Experiment Class Worksheet Baking Soda and Vinagar Experiment Baking Soda And Vinegar Lab Answers the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. a gas that was not created, but came out of the baking soda particles during the reaction with vinegar (co2) The balanced chemical equation is: Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2. Baking Soda And Vinegar Lab Answers.
From www.chegg.com
Solved Titration of vinegar lab report! I am providing the Baking Soda And Vinegar Lab Answers what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from. Baking Soda And Vinegar Lab Answers.
From www.worksheeto.com
14 Best Images of Baking Worksheets For Students Recipe Worksheets Baking Soda And Vinegar Lab Answers one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. O put the balloon over the mouth of. a gas that was not created, but came out of the baking soda particles during the reaction. Baking Soda And Vinegar Lab Answers.
From www.chegg.com
Solved Data Sheet and PostLab Questions Submit only pages Baking Soda And Vinegar Lab Answers Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. The balanced chemical equation is: the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. O put in 30 ml of vinegar in the bottle. what happens when. Baking Soda And Vinegar Lab Answers.
From www.chegg.com
Solved Stoichiometry Lab OBJECTIVES In this experiment you Baking Soda And Vinegar Lab Answers Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar.. Baking Soda And Vinegar Lab Answers.
From dxoowrfrf.blob.core.windows.net
Baking Soda And Vinegar Chemistry Lab at Andrea Doherty blog Baking Soda And Vinegar Lab Answers Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide +. Baking Soda And Vinegar Lab Answers.
From studylib.net
Baking Soda Vinegar Lab Write Up Baking Soda And Vinegar Lab Answers O put the balloon over the mouth of. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. o put a heaping spoonful of baking soda in the balloon. a gas that was not created, but came out of the baking soda particles during the. Baking Soda And Vinegar Lab Answers.
From www.chegg.com
Solved Stoichiometry with Kitchen Chemistry nb, you will be Baking Soda And Vinegar Lab Answers what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. O put in 30 ml of vinegar in the bottle. The balanced. Baking Soda And Vinegar Lab Answers.
From educationpossible.com
Baking Soda and Vinegar Balloon Experiment Education Possible Baking Soda And Vinegar Lab Answers Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: O put in 30 ml of vinegar in the bottle. a. Baking Soda And Vinegar Lab Answers.
From sydney-study-exams.blogspot.com
Stoichiometry Lab Vinegar And Baking Soda Answer Key 49+ Pages Solution Baking Soda And Vinegar Lab Answers one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: . Baking Soda And Vinegar Lab Answers.
From davida.davivienda.com
Baking Soda Vinegar Balloon Experiment Worksheet Printable Word Searches Baking Soda And Vinegar Lab Answers O put in 30 ml of vinegar in the bottle. O put the balloon over the mouth of. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. . Baking Soda And Vinegar Lab Answers.
From www.chegg.com
Solved Submit only pages 68 to Blackboard Table 1. Baking Soda And Vinegar Lab Answers O put the balloon over the mouth of. a gas that was not created, but came out of the baking soda particles during the reaction with vinegar (co2) the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. what happens when vinegar reacts with baking soda occurs in two steps,. Baking Soda And Vinegar Lab Answers.
From studylib.net
BAKING SODA AND VINEGAR INVESTIGATIONS GUIDED INQUIRY VERSION LAB 2 Baking Soda And Vinegar Lab Answers Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion O put in 30 ml of vinegar in the bottle. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole. Baking Soda And Vinegar Lab Answers.
From www.slideshare.net
Baking soda and vinegar experiment report Baking Soda And Vinegar Lab Answers a gas that was not created, but came out of the baking soda particles during the reaction with vinegar (co2) O put in 30 ml of vinegar in the bottle. The balanced chemical equation is: Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion o put a heaping. Baking Soda And Vinegar Lab Answers.
From shizenmedicare.com
nečistý Margaret Mitchell efektívna why does baking soda and vinegar Baking Soda And Vinegar Lab Answers what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: O put in 30 ml of vinegar in the bottle. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one. Baking Soda And Vinegar Lab Answers.
From www.youtube.com
Vinegar and Baking Soda Stoichiometry Lab YouTube Baking Soda And Vinegar Lab Answers what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. . Baking Soda And Vinegar Lab Answers.
From studylib.net
Stoichiometry and Baking Soda Lab Baking Soda And Vinegar Lab Answers The balanced chemical equation is: the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. O put in 30 ml of vinegar in the bottle. O put the balloon over the mouth of. a gas that was not created, but came out of the baking soda particles during the reaction with. Baking Soda And Vinegar Lab Answers.
From colouremployer8.gitlab.io
Unique Chemical Equation Of Vinegar And Baking Soda Electrostatic Notes Baking Soda And Vinegar Lab Answers o put a heaping spoonful of baking soda in the balloon. The balanced chemical equation is: Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion a gas that was not created, but came out of the baking soda particles during the reaction with vinegar (co2) the vinegar. Baking Soda And Vinegar Lab Answers.
From www.alamy.com
Science experiment with baking soda and vinegar balloon illustration Baking Soda And Vinegar Lab Answers the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. O put the balloon over the mouth of.. Baking Soda And Vinegar Lab Answers.
From worksheets.clipart-library.com
Vinegar and Baking Soda Stoichiometry Lab To predict Baking Soda And Vinegar Lab Answers The balanced chemical equation is: Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion a gas that was not created, but came out of the baking soda particles during the reaction with vinegar (co2) one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid. Baking Soda And Vinegar Lab Answers.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Baking Soda And Vinegar Lab Answers one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. O put in 30 ml of vinegar in the bottle. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2. Baking Soda And Vinegar Lab Answers.
From www.vecteezy.com
Baking Soda and Vinegar Balloon Science experiment, Chemistry Baking Soda And Vinegar Lab Answers Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion The balanced chemical equation is: what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: O put the balloon over the mouth of. o put. Baking Soda And Vinegar Lab Answers.
From studylib.net
Vinegar and Baking Soda Investigation Baking Soda And Vinegar Lab Answers O put the balloon over the mouth of. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: The balanced chemical equation is: a gas. Baking Soda And Vinegar Lab Answers.
From brainly.com
baking soda and vinegar lab demo part 2 mass! 7th grade please help Baking Soda And Vinegar Lab Answers O put in 30 ml of vinegar in the bottle. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. a gas that was not created, but came out of the baking soda particles during the reaction with vinegar (co2) Baking soda (sodium bicarbonate) plus vinegar. Baking Soda And Vinegar Lab Answers.
From www.slideserve.com
PPT Baking Soda and Vinegar Limiting Reactant Lab PowerPoint Baking Soda And Vinegar Lab Answers Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: The balanced chemical equation is: O put in 30 ml of vinegar in the bottle. Nahco. Baking Soda And Vinegar Lab Answers.
From worksheetonironautica5p.z13.web.core.windows.net
Experiments Using Vinegar And Baking Soda Baking Soda And Vinegar Lab Answers Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. o put a heaping spoonful of baking soda in the balloon. Baking soda (sodium bicarbonate) plus vinegar (acetic. Baking Soda And Vinegar Lab Answers.
From studylib.net
Baking Soda and Vinegar (Limiting Reactants) Lab Baking Soda And Vinegar Lab Answers Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: O put in 30 ml of vinegar in the bottle. Nahco 3 + hc 2 h. Baking Soda And Vinegar Lab Answers.
From www.numerade.com
SOLVED Baking soda and vinegar react according to the following Baking Soda And Vinegar Lab Answers Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion O put in 30 ml of vinegar in the bottle. o put a heaping spoonful of baking soda in the balloon. the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. O. Baking Soda And Vinegar Lab Answers.
From www.youtube.com
Baking soda and vinegar reaction. ⚗️ 3 Experiments to do with kids ⚗️ Baking Soda And Vinegar Lab Answers The balanced chemical equation is: O put in 30 ml of vinegar in the bottle. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. o put a heaping spoonful of baking soda in the balloon. a gas that was not created, but came out. Baking Soda And Vinegar Lab Answers.
From www.slideserve.com
PPT Baking Soda/Vinegar Stoichiometry Lab PowerPoint Presentation Baking Soda And Vinegar Lab Answers one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: The. Baking Soda And Vinegar Lab Answers.
From worksheets.clipart-library.com
Vinegar and Baking Soda Stoichiometry Lab To predict Baking Soda And Vinegar Lab Answers Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion + acetate ion the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. The balanced chemical equation is: O put the balloon over the mouth of. O put in 30 ml of vinegar in the bottle.. Baking Soda And Vinegar Lab Answers.
From stoichiometricequiv.blogspot.com
The Stoichiometric Equivalent Baking Soda Lab Baking Soda And Vinegar Lab Answers Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. o put a heaping spoonful of baking soda in the balloon. O put the balloon over the mouth of. Baking soda (sodium bicarbonate) plus vinegar (acetic acid) yields carbon dioxide + water + sodium ion +. Baking Soda And Vinegar Lab Answers.
From www.studocu.com
D The Investigation of Carbon Dixode In A Baking Soda and Vinegar Lab Baking Soda And Vinegar Lab Answers o put a heaping spoonful of baking soda in the balloon. the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one. Baking Soda And Vinegar Lab Answers.
From printabletryledura.z14.web.core.windows.net
Science Experiments Baking Soda And Vinegar Baking Soda And Vinegar Lab Answers what happens when vinegar reacts with baking soda occurs in two steps, but the overall process can be summarized by the following word equation: one mole of sodium bicarbonate (baking soda) reacts with one mole of acetic acid (from vinegar) to yield one mole of sodium acetate, one mole of water, and one mole of carbon dioxide. . Baking Soda And Vinegar Lab Answers.
From worksheets.clipart-library.com
Solved Vinegar and Baking Soda Stoichiometry Lalb Purpose Baking Soda And Vinegar Lab Answers o put a heaping spoonful of baking soda in the balloon. Nahco 3 + hc 2 h 3 o 2 → nac 2 h 3 o 2 + h 2 o + co 2. The balanced chemical equation is: the vinegar and baking soda experiment is a classic science experiment that involves a reaction between vinegar. Baking soda. Baking Soda And Vinegar Lab Answers.