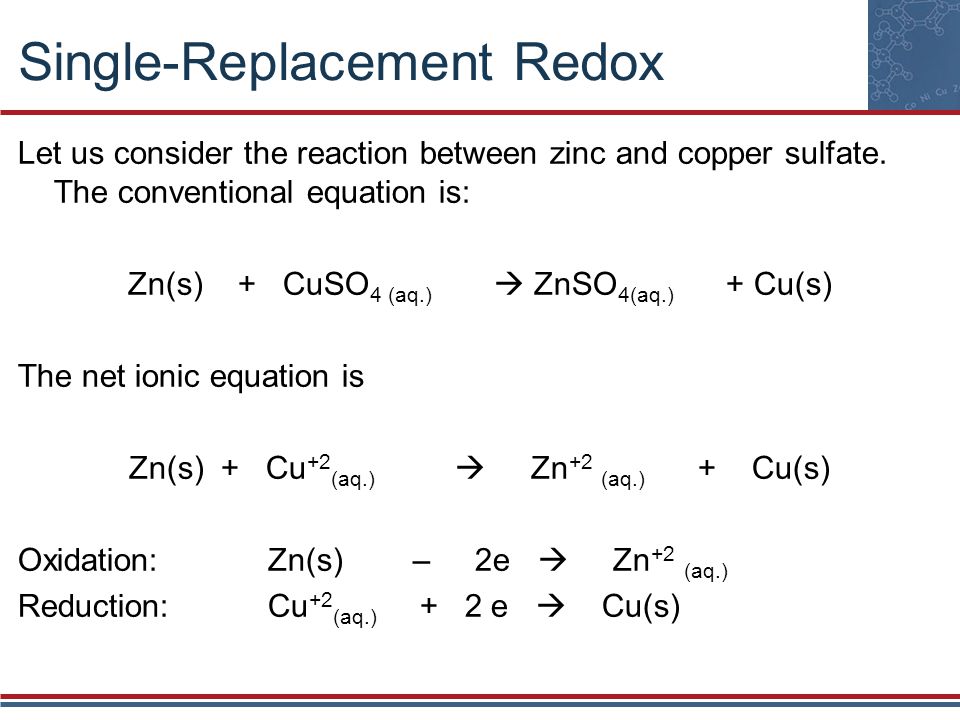
Zinc And Copper Ii Nitrate . Zinc is a more active metal than copper (it lies higher up the activity series). Place the strip of zinc in the zinc nitrate solution. the net reaction is the oxidation of zinc by copper(ii) ions: this is because copper is more reactive than silver, so it can displace silver from silver nitrate. Copper + silver nitrate → copper(ii). use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. However, in the second reaction, the zinc. when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. Place the strip of copper in the copper sulphate solution. \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place in separate locations: in the first reaction, the copper ion is able to oxidize the zinc metal.
from schoolworkhelper.net
Place the strip of copper in the copper sulphate solution. However, in the second reaction, the zinc. in the first reaction, the copper ion is able to oxidize the zinc metal. when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. Copper + silver nitrate → copper(ii). Zinc is a more active metal than copper (it lies higher up the activity series). the net reaction is the oxidation of zinc by copper(ii) ions: this is because copper is more reactive than silver, so it can displace silver from silver nitrate. \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place in separate locations: use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc.
Single Displacement Reactions Lab Explained SchoolWorkHelper
Zinc And Copper Ii Nitrate this is because copper is more reactive than silver, so it can displace silver from silver nitrate. Place the strip of copper in the copper sulphate solution. this is because copper is more reactive than silver, so it can displace silver from silver nitrate. \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place in separate locations: Zinc is a more active metal than copper (it lies higher up the activity series). use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. However, in the second reaction, the zinc. Place the strip of zinc in the zinc nitrate solution. when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. in the first reaction, the copper ion is able to oxidize the zinc metal. the net reaction is the oxidation of zinc by copper(ii) ions: Copper + silver nitrate → copper(ii).
From www.numerade.com
SOLVED 2a. Zinc metal reacts with silver nitrate ro form silver metal Zinc And Copper Ii Nitrate use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. Place the strip of copper in the copper sulphate solution. \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place in separate locations: Copper + silver nitrate → copper(ii). the net. Zinc And Copper Ii Nitrate.
From dxomzyoyw.blob.core.windows.net
Zinc Nitrate And Magnesium Reaction at Amanda Fulton blog Zinc And Copper Ii Nitrate However, in the second reaction, the zinc. Place the strip of copper in the copper sulphate solution. when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. the net reaction is the oxidation of zinc by copper(ii) ions: Zinc is a more active metal than copper (it. Zinc And Copper Ii Nitrate.
From www.vectorstock.com
Zinc nitrate znn2o6 molecule Royalty Free Vector Image Zinc And Copper Ii Nitrate Place the strip of copper in the copper sulphate solution. Place the strip of zinc in the zinc nitrate solution. Copper + silver nitrate → copper(ii). in the first reaction, the copper ion is able to oxidize the zinc metal. Zinc is a more active metal than copper (it lies higher up the activity series). the net reaction. Zinc And Copper Ii Nitrate.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs Zinc And Copper Ii Nitrate the net reaction is the oxidation of zinc by copper(ii) ions: However, in the second reaction, the zinc. when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. this is because copper is more reactive than silver, so it can displace silver from silver nitrate. Place. Zinc And Copper Ii Nitrate.
From 2012books.lardbucket.org
Describing Electrochemical Cells Zinc And Copper Ii Nitrate However, in the second reaction, the zinc. when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. Place the strip of copper in the copper sulphate solution. the net reaction is the oxidation of zinc by copper(ii) ions: Copper + silver nitrate → copper(ii). \[zn_{(s)} + cu^{2+}. Zinc And Copper Ii Nitrate.
From www.numerade.com
SOLVED A student adds 10.0 g of zinc to an aqueous solution containing Zinc And Copper Ii Nitrate \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place in separate locations: Zinc is a more active metal than copper (it lies higher up the activity series). Copper + silver nitrate → copper(ii). this is because copper is more reactive than silver, so it can displace silver from silver. Zinc And Copper Ii Nitrate.
From studylib.net
zinc nitrate Zinc And Copper Ii Nitrate Copper + silver nitrate → copper(ii). Place the strip of zinc in the zinc nitrate solution. \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place in separate locations: Zinc is a more active metal than copper (it lies higher up the activity series). in the first reaction, the copper. Zinc And Copper Ii Nitrate.
From www.chegg.com
Solved Zinc and lead (II) nitrate react to form zinc nitrate Zinc And Copper Ii Nitrate this is because copper is more reactive than silver, so it can displace silver from silver nitrate. when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. in the first reaction, the copper ion is able to oxidize the zinc metal. Place the strip of zinc. Zinc And Copper Ii Nitrate.
From www.toppr.com
3. (a) Write the symbol with charge of the radicals present and than wo Zinc And Copper Ii Nitrate Place the strip of zinc in the zinc nitrate solution. Zinc is a more active metal than copper (it lies higher up the activity series). Place the strip of copper in the copper sulphate solution. the net reaction is the oxidation of zinc by copper(ii) ions: when you add a strip of zinc metal to a solution of. Zinc And Copper Ii Nitrate.
From www.chegg.com
Solved 1) Zinc and lead (II) nitrate react to form zinc Zinc And Copper Ii Nitrate Place the strip of zinc in the zinc nitrate solution. this is because copper is more reactive than silver, so it can displace silver from silver nitrate. Copper + silver nitrate → copper(ii). Zinc is a more active metal than copper (it lies higher up the activity series). However, in the second reaction, the zinc. in the first. Zinc And Copper Ii Nitrate.
From www.coursehero.com
[Solved] Sketch a diagram of a copper/zinc Daniell cell. Label all the Zinc And Copper Ii Nitrate in the first reaction, the copper ion is able to oxidize the zinc metal. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. Place the strip of zinc in the zinc nitrate solution. Copper + silver nitrate → copper(ii). this is because copper is more reactive than silver,. Zinc And Copper Ii Nitrate.
From www.youtube.com
Zinc nitrate YouTube Zinc And Copper Ii Nitrate in the first reaction, the copper ion is able to oxidize the zinc metal. Place the strip of copper in the copper sulphate solution. this is because copper is more reactive than silver, so it can displace silver from silver nitrate. the net reaction is the oxidation of zinc by copper(ii) ions: \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+}. Zinc And Copper Ii Nitrate.
From www.youtube.com
Zinc and Copper II Chloride YouTube Zinc And Copper Ii Nitrate when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. Zinc is a more active metal than copper (it lies higher up the activity series). this is because copper is more reactive than silver, so it can displace silver from silver nitrate. in the first reaction,. Zinc And Copper Ii Nitrate.
From www.youtube.com
How to Write the Formula for Zinc nitrate YouTube Zinc And Copper Ii Nitrate Place the strip of zinc in the zinc nitrate solution. Place the strip of copper in the copper sulphate solution. in the first reaction, the copper ion is able to oxidize the zinc metal. Copper + silver nitrate → copper(ii). the net reaction is the oxidation of zinc by copper(ii) ions: use cell notation to describe the. Zinc And Copper Ii Nitrate.
From www.youtube.com
AgNO3+Zn→Ag +Zn(NO3)2 Balanced EquationSilver nitrate+Zinc =Silver Zinc And Copper Ii Nitrate in the first reaction, the copper ion is able to oxidize the zinc metal. when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. Copper + silver nitrate → copper(ii). the net reaction is the oxidation of zinc by copper(ii) ions: \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+}. Zinc And Copper Ii Nitrate.
From www.youtube.com
Lead Nitrate + Zinc YouTube Zinc And Copper Ii Nitrate However, in the second reaction, the zinc. Place the strip of zinc in the zinc nitrate solution. \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place in separate locations: this is because copper is more reactive than silver, so it can displace silver from silver nitrate. the net. Zinc And Copper Ii Nitrate.
From www.chegg.com
Solved (6pts) Reaction A Zinc Metal, Zn(s), and Copper(II) Zinc And Copper Ii Nitrate use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. in the first reaction, the copper ion is able to oxidize the zinc metal. Place the strip of copper in the copper sulphate solution. Place the strip of zinc in the zinc nitrate solution. Zinc is a more active metal. Zinc And Copper Ii Nitrate.
From www.youtube.com
Zinc Nitrate + Lead YouTube Zinc And Copper Ii Nitrate \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place in separate locations: in the first reaction, the copper ion is able to oxidize the zinc metal. this is because copper is more reactive than silver, so it can displace silver from silver nitrate. Place the strip of zinc. Zinc And Copper Ii Nitrate.
From www.slideserve.com
PPT Balancing Chemical Reactions PowerPoint Presentation, free Zinc And Copper Ii Nitrate use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. Copper + silver nitrate → copper(ii). this is because copper is more reactive than silver, so it can displace silver from silver nitrate. Place the strip of zinc in the zinc nitrate solution. Place the strip of copper in the. Zinc And Copper Ii Nitrate.
From www.youtube.com
Zinc + Copper Sulfate Reaction YouTube Zinc And Copper Ii Nitrate this is because copper is more reactive than silver, so it can displace silver from silver nitrate. Copper + silver nitrate → copper(ii). Place the strip of copper in the copper sulphate solution. Place the strip of zinc in the zinc nitrate solution. Zinc is a more active metal than copper (it lies higher up the activity series). . Zinc And Copper Ii Nitrate.
From www.youtube.com
Zinc Nitrate + Copper YouTube Zinc And Copper Ii Nitrate However, in the second reaction, the zinc. the net reaction is the oxidation of zinc by copper(ii) ions: use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. Zinc is a more active metal than copper (it lies higher up the activity series). Place the strip of copper in the. Zinc And Copper Ii Nitrate.
From www.coursehero.com
[Solved] System 6 aqueous zinc nitrate and aqueous chromium(II Zinc And Copper Ii Nitrate Copper + silver nitrate → copper(ii). use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. in the first reaction, the copper ion is able to oxidize the zinc metal. this is because copper is more reactive than silver, so it can displace silver from silver nitrate. Place the. Zinc And Copper Ii Nitrate.
From www.slideserve.com
PPT Chapter 18 Electrochemistry PowerPoint Presentation, free Zinc And Copper Ii Nitrate the net reaction is the oxidation of zinc by copper(ii) ions: use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. Place the strip of zinc in the zinc nitrate solution. in the first reaction, the copper ion is able to oxidize the zinc metal. Copper + silver nitrate. Zinc And Copper Ii Nitrate.
From www.youtube.com
Displacement reaction of zinc and copper(II) oxide YouTube Zinc And Copper Ii Nitrate when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. Place the strip of zinc in the zinc nitrate solution. Copper + silver nitrate → copper(ii). use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. in the. Zinc And Copper Ii Nitrate.
From schoolworkhelper.net
Single Displacement Reactions Lab Explained SchoolWorkHelper Zinc And Copper Ii Nitrate Place the strip of zinc in the zinc nitrate solution. Zinc is a more active metal than copper (it lies higher up the activity series). this is because copper is more reactive than silver, so it can displace silver from silver nitrate. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal. Zinc And Copper Ii Nitrate.
From www.youtube.com
Reaction 12 Zinc and Copper (II) Nitrate YouTube Zinc And Copper Ii Nitrate Copper + silver nitrate → copper(ii). this is because copper is more reactive than silver, so it can displace silver from silver nitrate. Place the strip of zinc in the zinc nitrate solution. \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place in separate locations: However, in the second. Zinc And Copper Ii Nitrate.
From www.mbizmarket.co.id
(Zinc Nitrate Hexahydrate) Zn(NH3)2.6H2O Zinc And Copper Ii Nitrate the net reaction is the oxidation of zinc by copper(ii) ions: when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. Place the strip of zinc in the zinc nitrate solution. use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper. Zinc And Copper Ii Nitrate.
From www.youtube.com
Reaction of Copper with Zinc and Magnesium Nitrate YouTube Zinc And Copper Ii Nitrate use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place. Zinc And Copper Ii Nitrate.
From ramonnewscook.blogspot.com
Zinc Nitrate + Sodium Carbonate Balanced Equation Zinc And Copper Ii Nitrate Copper + silver nitrate → copper(ii). this is because copper is more reactive than silver, so it can displace silver from silver nitrate. the net reaction is the oxidation of zinc by copper(ii) ions: when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. Zinc is. Zinc And Copper Ii Nitrate.
From www.youtube.com
[4K] Displacement Reaction of Metals Zinc in Copper (II) Sulfate Zinc And Copper Ii Nitrate However, in the second reaction, the zinc. the net reaction is the oxidation of zinc by copper(ii) ions: Zinc is a more active metal than copper (it lies higher up the activity series). in the first reaction, the copper ion is able to oxidize the zinc metal. Place the strip of copper in the copper sulphate solution. . Zinc And Copper Ii Nitrate.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs Zinc And Copper Ii Nitrate in the first reaction, the copper ion is able to oxidize the zinc metal. when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place in separate locations: However,. Zinc And Copper Ii Nitrate.
From fphoto.photoshelter.com
science chemistry redox reaction zinc copper Fundamental Photographs Zinc And Copper Ii Nitrate in the first reaction, the copper ion is able to oxidize the zinc metal. However, in the second reaction, the zinc. when you add a strip of zinc metal to a solution of copper (ii) nitrate, a single replacement reaction takes place. the net reaction is the oxidation of zinc by copper(ii) ions: \[zn_{(s)} + cu^{2+} \rightarrow. Zinc And Copper Ii Nitrate.
From www.nanochemazone.com
Zinc Nitrate Hexahydrate Nanochemazone CAS 10196186 Zinc And Copper Ii Nitrate Zinc is a more active metal than copper (it lies higher up the activity series). use cell notation to describe the galvanic cell where copper(ii) ions are reduced to copper metal and zinc. Place the strip of zinc in the zinc nitrate solution. this is because copper is more reactive than silver, so it can displace silver from. Zinc And Copper Ii Nitrate.
From www.youtube.com
WHEN ZINC NITRATE REACTS WITH MAGNESIUM RIBBON YouTube Zinc And Copper Ii Nitrate \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions) take place in separate locations: Zinc is a more active metal than copper (it lies higher up the activity series). in the first reaction, the copper ion is able to oxidize the zinc metal. Copper + silver nitrate → copper(ii). use. Zinc And Copper Ii Nitrate.
From www.youtube.com
Equation for Zn(NO3)2 + H2O (Zinc nitrate + Water) YouTube Zinc And Copper Ii Nitrate Zinc is a more active metal than copper (it lies higher up the activity series). this is because copper is more reactive than silver, so it can displace silver from silver nitrate. Place the strip of zinc in the zinc nitrate solution. \[zn_{(s)} + cu^{2+} \rightarrow zn^{2+} + cu_{(s)}\] but this time, the oxidation and reduction steps (half reactions). Zinc And Copper Ii Nitrate.