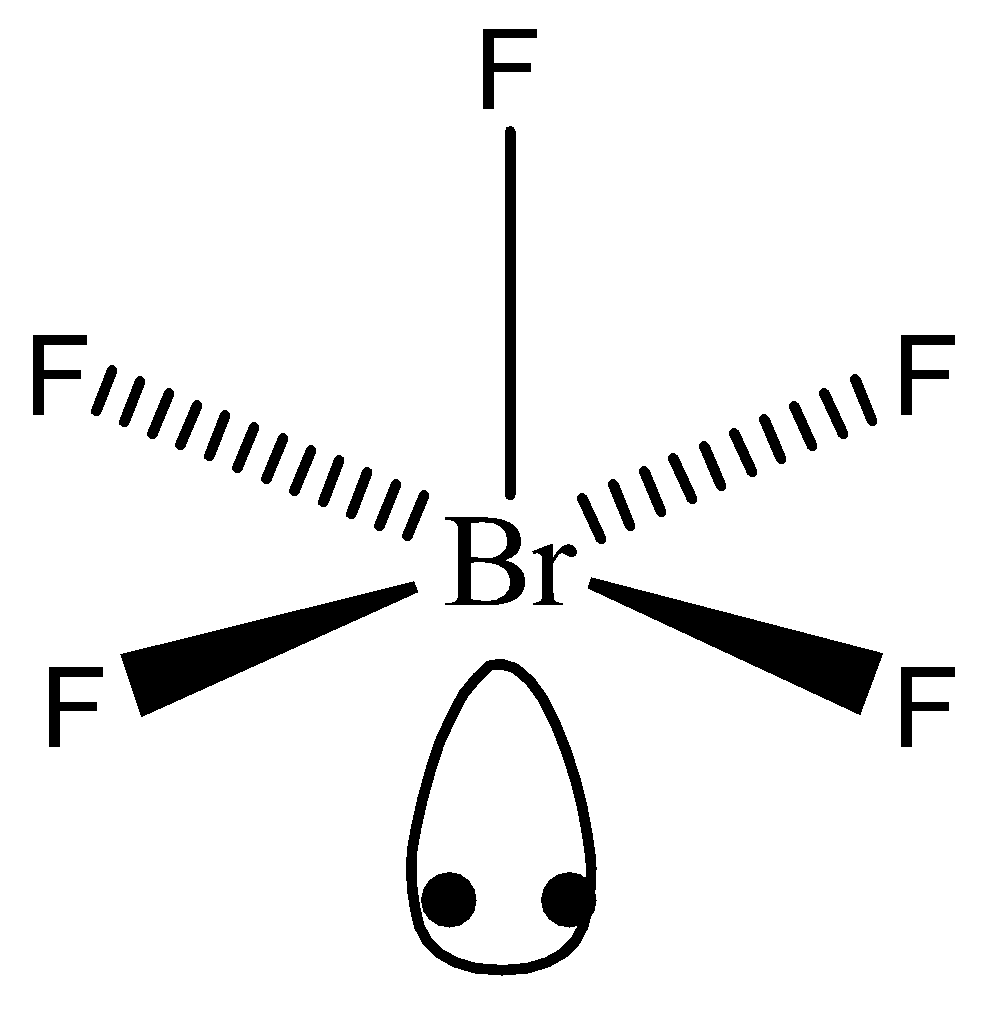
Bromine Pentafluoride Chemical Equation . It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Bromine pentafluoride is a fluoride of bromine. Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. It is an interhalogen compound uses as a fluorinating reagent. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Bromine pentafluoride = dibromine + difluorine. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent. It is used in various rockets and chemicals.
from www.vedantu.com
It is used in various rockets and chemicals. Bromine pentafluoride = dibromine + difluorine. Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. Bromine pentafluoride is a fluoride of bromine. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. It is a strong fluorinating agent. It is an interhalogen compound uses as a fluorinating reagent. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine.
What is the geometry of the molecule of bromine pentafluoride?A.Square
Bromine Pentafluoride Chemical Equation Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is an interhalogen compound uses as a fluorinating reagent. It is used in various rockets and chemicals. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Bromine pentafluoride = dibromine + difluorine. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent. Bromine pentafluoride is a fluoride of bromine. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5.
From www.wikiwand.com
Brompentafluorid Wikiwand Bromine Pentafluoride Chemical Equation Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. Bromine pentafluoride = dibromine + difluorine. It is an interhalogen compound uses as a fluorinating reagent. It is a strong fluorinating agent. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Note, members of the same family tend to form similar compounds,. Bromine Pentafluoride Chemical Equation.
From cartoondealer.com
Elemental Bromine Br2, Molecule. Skeletal Formula. Chemical Structure Bromine Pentafluoride Chemical Equation It is an interhalogen compound uses as a fluorinating reagent. Bromine pentafluoride is a fluoride of bromine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Bromine pentafluoride = dibromine + difluorine. Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. Bromine. Bromine Pentafluoride Chemical Equation.
From baike.baidu.hk
五氟化溴_百度百科 Bromine Pentafluoride Chemical Equation Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. It is used in various rockets and chemicals. It is an interhalogen compound uses as a fluorinating reagent. It is a strong fluorinating agent. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of. Bromine Pentafluoride Chemical Equation.
From ajorpng.blogspot.com
Chlorine Pentafluoride Molecular Geometry / De wikipedia, la Bromine Pentafluoride Chemical Equation Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent. It is used in various rockets and chemicals. It is an interhalogen compound uses as a fluorinating reagent. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Brf5. Bromine Pentafluoride Chemical Equation.
From ajorpng.blogspot.com
Chlorine Pentafluoride Electron Pair Geometry Brf5 or bromine Bromine Pentafluoride Chemical Equation It is used in various rockets and chemicals. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is an interhalogen compound uses as a fluorinating reagent. Bromine pentafluoride is a fluoride of bromine. It is a strong fluorinating agent. Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. Note, members. Bromine Pentafluoride Chemical Equation.
From exodfsivt.blob.core.windows.net
Bromine Pentafluoride Formula at Lawrence Manzi blog Bromine Pentafluoride Chemical Equation Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is an interhalogen compound uses as a fluorinating reagent. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure. Bromine Pentafluoride Chemical Equation.
From ajorpng.blogspot.com
Chlorine Pentafluoride Electron Pair Geometry Brf5 or bromine Bromine Pentafluoride Chemical Equation It is a strong fluorinating agent. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. It is an interhalogen compound uses as a fluorinating reagent. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the.. Bromine Pentafluoride Chemical Equation.
From www.numerade.com
SOLVED (d) carbon and bromine ionic molecular ionic compound name Bromine Pentafluoride Chemical Equation Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. It is a strong fluorinating agent. Bromine pentafluoride is a fluoride of bromine. Bromine pentafluoride, br f 5, is an interhalogen compound and. Bromine Pentafluoride Chemical Equation.
From imgbin.com
Interhalogen Bromine Pentafluoride Chlorine Trifluoride Iodine Bromine Pentafluoride Chemical Equation Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. Bromine pentafluoride is a fluoride of bromine. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. It is an interhalogen compound uses as a fluorinating reagent. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is. Bromine Pentafluoride Chemical Equation.
From alchetron.com
Bromine pentafluoride Alchetron, The Free Social Encyclopedia Bromine Pentafluoride Chemical Equation It is used in various rockets and chemicals. It is a strong fluorinating agent. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Bromine pentafluoride = dibromine +. Bromine Pentafluoride Chemical Equation.
From www.dreamstime.com
BrF5 Bromine Pentafluoride Stock Image Image of brf5, container Bromine Pentafluoride Chemical Equation Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. It is a strong fluorinating agent. Bromine pentafluoride is a fluoride of bromine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to. Bromine Pentafluoride Chemical Equation.
From www.numerade.com
SOLVEDPractice problems Balance the following equations KBr Fe(OH Bromine Pentafluoride Chemical Equation It is used in various rockets and chemicals. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Bromine pentafluoride is a fluoride of bromine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Brf5. Bromine Pentafluoride Chemical Equation.
From chemistry-europe.onlinelibrary.wiley.com
Bromine Pentafluoride BrF5, the Formation of [BrF6]− Salts, and the Bromine Pentafluoride Chemical Equation Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. Bromine pentafluoride is a fluoride of bromine. It is used in various rockets and chemicals. It is a strong fluorinating agent. Note, members of the same family tend to form similar compounds, so. Bromine Pentafluoride Chemical Equation.
From www.youtube.com
AP08.10 Lewis Dot structure bromine pentafluoride BrF5 YouTube Bromine Pentafluoride Chemical Equation Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Bromine pentafluoride = dibromine + difluorine. It is a strong fluorinating agent. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Bromine pentafluoride, br f 5, is an interhalogen compound and a. Bromine Pentafluoride Chemical Equation.
From www.bartleby.com
Answered Describe the steps you would take to… bartleby Bromine Pentafluoride Chemical Equation It is a strong fluorinating agent. It is an interhalogen compound uses as a fluorinating reagent. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is used in various rockets and chemicals. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Bromine pentafluoride is a fluoride of bromine. Bromine. Bromine Pentafluoride Chemical Equation.
From www.numerade.com
SOLVED Aqueous sodium chloride (NaCl) and liquid bromine (Brz are Bromine Pentafluoride Chemical Equation Bromine pentafluoride is a fluoride of bromine. It is used in various rockets and chemicals. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Bromine pentafluoride = dibromine + difluorine. Note, members of the. Bromine Pentafluoride Chemical Equation.
From askfilo.com
What is the geometry of molecule of bromine pentafluoride? [MH CET 2014].. Bromine Pentafluoride Chemical Equation Bromine pentafluoride is a fluoride of bromine. It is a strong fluorinating agent. Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. Bromine pentafluoride = dibromine + difluorine. It is used in various rockets and chemicals. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine. Bromine Pentafluoride Chemical Equation.
From digital.library.unt.edu
Refractive Indices Of The Systems Uranium HexafluorideBromine Bromine Pentafluoride Chemical Equation Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. It is used in various rockets and chemicals. Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. It is. Bromine Pentafluoride Chemical Equation.
From gbu-taganskij.ru
Elemental Bromine Br2, Skeletal Formula Stock Vector , 55 OFF Bromine Pentafluoride Chemical Equation It is used in various rockets and chemicals. Bromine pentafluoride is a fluoride of bromine. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Brf5. Bromine Pentafluoride Chemical Equation.
From gbu-taganskij.ru
BrF5 (Bromine Pentafluoride) Molecular Geometry, Bond, 53 OFF Bromine Pentafluoride Chemical Equation Bromine pentafluoride = dibromine + difluorine. It is an interhalogen compound uses as a fluorinating reagent. It is a strong fluorinating agent. Bromine pentafluoride is a fluoride of bromine. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Note, members of the same family tend to form similar. Bromine Pentafluoride Chemical Equation.
From es.vecteezy.com
símbolo de bromo elemento químico de la tabla periódica. ilustración Bromine Pentafluoride Chemical Equation Bromine pentafluoride is a fluoride of bromine. It is used in various rockets and chemicals. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. It is an interhalogen compound uses as a fluorinating reagent. It is a strong fluorinating agent. Note, members of the same family tend to. Bromine Pentafluoride Chemical Equation.
From www.vrogue.co
What Is The Hybridization Of Bromine Pentafluoride vrogue.co Bromine Pentafluoride Chemical Equation It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is an interhalogen compound uses as a fluorinating reagent. It is a strong fluorinating agent. It is used in various rockets and chemicals. Bromine. Bromine Pentafluoride Chemical Equation.
From www.youtube.com
How to Write the Formula for Bromine Liquid YouTube Bromine Pentafluoride Chemical Equation Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. It is a strong fluorinating agent. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. It is used. Bromine Pentafluoride Chemical Equation.
From www.anyrgb.com
Bromine pentafluoride, phosphorus Tribromide, Nitrogen trichloride Bromine Pentafluoride Chemical Equation It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Bromine pentafluoride is a fluoride of bromine. Bromine pentafluoride = dibromine + difluorine. Bromine pentafluoride is a pale yellow color liquid with the chemical formula. Bromine Pentafluoride Chemical Equation.
From www.vedantu.com
What is the geometry of the molecule of bromine pentafluoride?A.Square Bromine Pentafluoride Chemical Equation Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. It is used in various rockets and chemicals. It is a strong fluorinating agent. It is used in oxygen isotope analysis, as an. Bromine Pentafluoride Chemical Equation.
From www.alamy.com
BrF5 Bromine Pentafluoride. Chemical in a metal container Stock Photo Bromine Pentafluoride Chemical Equation It is an interhalogen compound uses as a fluorinating reagent. It is used in various rockets and chemicals. Bromine pentafluoride = dibromine + difluorine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. It is a strong fluorinating agent. Bromine pentafluoride is a pale yellow color. Bromine Pentafluoride Chemical Equation.
From www.chegg.com
Solved The pale yellow liquid bromine pentafluoride reacts Bromine Pentafluoride Chemical Equation It is an interhalogen compound uses as a fluorinating reagent. It is a strong fluorinating agent. It is used in various rockets and chemicals. Bromine pentafluoride is a fluoride of bromine. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Note, members of the same family tend to. Bromine Pentafluoride Chemical Equation.
From www.numerade.com
SOLVED (ii) Aniline reacts with bromine water in aqueous medium (only Bromine Pentafluoride Chemical Equation Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. It is used in various rockets and chemicals. Bromine pentafluoride is a fluoride of bromine. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is an interhalogen compound uses as a fluorinating reagent. Note, members of the same family tend. Bromine Pentafluoride Chemical Equation.
From imgbin.com
Bromine Monofluoride Bromine Pentafluoride Bromine Trifluoride Chlorine Bromine Pentafluoride Chemical Equation It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Bromine pentafluoride = dibromine + difluorine. Bromine pentafluoride is a fluoride of bromine. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Note, members of the same family tend to form similar compounds, so. Bromine Pentafluoride Chemical Equation.
From in.pinterest.com
Bromine trifluoride has the chemical formula BrF3 and interhalogen Bromine Pentafluoride Chemical Equation It is a strong fluorinating agent. It is an interhalogen compound uses as a fluorinating reagent. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. It is used in various rockets and. Bromine Pentafluoride Chemical Equation.
From www.slideserve.com
PPT VSEPR and Chemical Polarity PowerPoint Presentation, free Bromine Pentafluoride Chemical Equation Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. It is used in various rockets and chemicals. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Bromine pentafluoride. Bromine Pentafluoride Chemical Equation.
From imgbin.com
Chlorine Trifluoride Chlorine Pentafluoride Boron Trifluoride Chlorine Bromine Pentafluoride Chemical Equation It is a strong fluorinating agent. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Note, members of the same family. Bromine Pentafluoride Chemical Equation.
From www.pinterest.com
PF5 Lewis Structure (Phosphorus Pentafluoride) Lewis, Phosphorus Bromine Pentafluoride Chemical Equation Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. It is used in various rockets and chemicals. It is a strong fluorinating agent. Brf5 = br2 + f2 is a decomposition reaction where two moles of bromine. Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions. Bromine Pentafluoride Chemical Equation.
From favpng.com
VSEPR Theory Chemistry Bromine Pentafluoride Electron Kugelwolkenmodell Bromine Pentafluoride Chemical Equation Note, members of the same family tend to form similar compounds, so bromine and iodine form similar anions to chlorine (see figure 2.7.6),. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Bromine pentafluoride is a fluoride of bromine. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as. Bromine Pentafluoride Chemical Equation.
From unacademy.com
What is the Hybridization of Bromine Pentafluoride Bromine Pentafluoride Chemical Equation Bromine pentafluoride is a pale yellow color liquid with the chemical formula brf5. Bromine pentafluoride = dibromine + difluorine. It is used in oxygen isotope analysis, as an oxidizer in liquid rocket propellants, and as a fluorinating agent in the. Bromine pentafluoride, br f 5, is an interhalogen compound and a fluoride of bromine. Brf5 = br2 + f2 is. Bromine Pentafluoride Chemical Equation.