Copper 2 Oxide Formation . Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. This process typically happens in the presence of water and oxygen in. A black solid, it is one of the two stable oxides of copper, the. The formation of copper oxide often results from the oxidation of copper. Copper (ii) oxide is made of. If they weigh the reactants and products. It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. The ‘ii’ in its name signifies that the copper ion. How do you write copper (ii) oxide?
from www.youtube.com
Copper (ii) oxide is made of. The ‘ii’ in its name signifies that the copper ion. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). A black solid, it is one of the two stable oxides of copper, the. How do you write copper (ii) oxide? The formation of copper oxide often results from the oxidation of copper. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. This process typically happens in the presence of water and oxygen in.
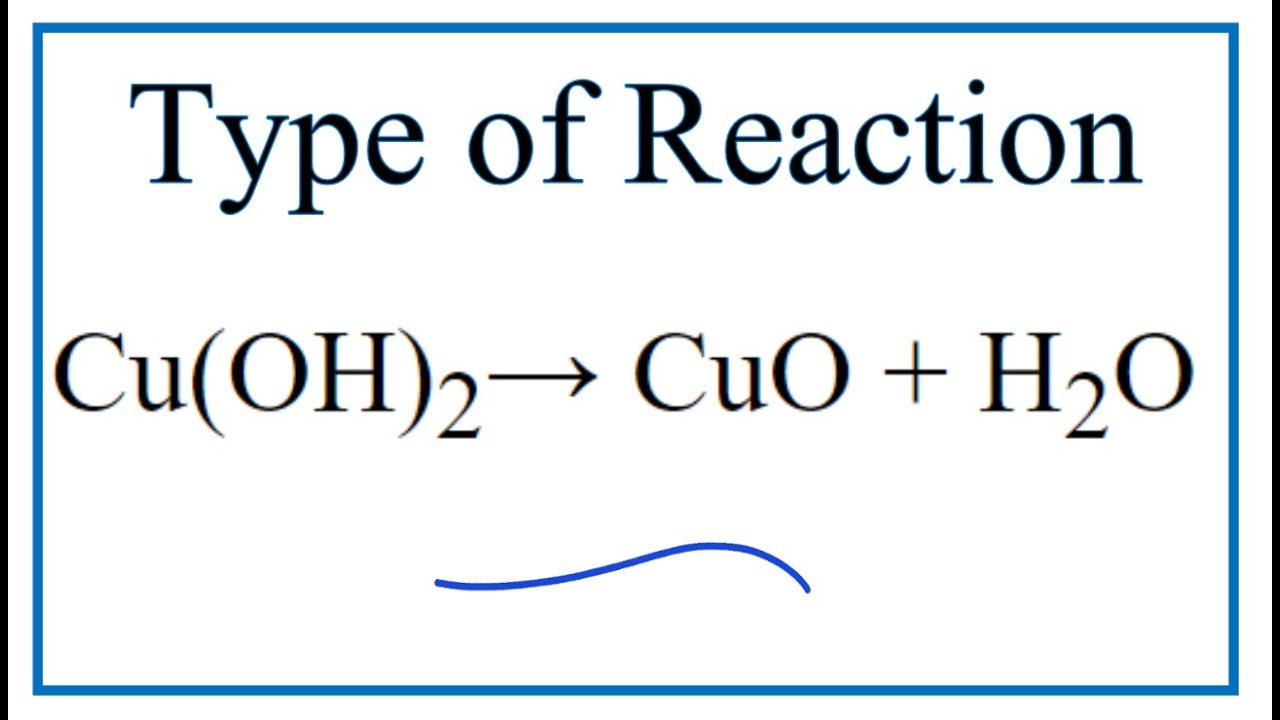
Type of Reaction for Cu(OH)2 = CuO + H2O YouTube
Copper 2 Oxide Formation The ‘ii’ in its name signifies that the copper ion. It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. The ‘ii’ in its name signifies that the copper ion. If they weigh the reactants and products. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. How do you write copper (ii) oxide? The formation of copper oxide often results from the oxidation of copper. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Copper (ii) oxide is made of. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. This process typically happens in the presence of water and oxygen in. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. A black solid, it is one of the two stable oxides of copper, the.
From www.toppr.com
9 Copper(t) carbonate is broken down by heating to form copper(11 Copper 2 Oxide Formation Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. The ‘ii’ in its name signifies that the copper ion. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). The formation of copper oxide often results from the oxidation of copper. In this experiment, students heat copper(ii) oxide. Copper 2 Oxide Formation.
From slideplayer.com
Properties of Matter Chapter 4 ppt download Copper 2 Oxide Formation It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. If they weigh the reactants and products. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. The ‘ii’ in its name signifies that the copper ion. This process typically happens in the presence of water. Copper 2 Oxide Formation.
From www.slideshare.net
Chapter 4 Copper 2 Oxide Formation In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. The formation of copper oxide often results from the oxidation of copper. A black solid, it is. Copper 2 Oxide Formation.
From www.researchgate.net
(a) TEM and SAED image of copper(II) oxide nanoparticles (b) HRTEM Copper 2 Oxide Formation Copper (ii) oxide is made of. The ‘ii’ in its name signifies that the copper ion. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. Copper(ii) oxide or cupric oxide. Copper 2 Oxide Formation.
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water Copper 2 Oxide Formation Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. The formation of copper. Copper 2 Oxide Formation.
From www.toppr.com
Write balanced chemical equations for the following word equation Copper 2 Oxide Formation As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). The formation of copper oxide often results from the oxidation of copper. How do you write copper (ii) oxide? If they weigh the reactants and products. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. A black solid,. Copper 2 Oxide Formation.
From www.youtube.com
Copper Oxide Synthesis YouTube Copper 2 Oxide Formation Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. A black solid, it is one of the two stable oxides of copper,. Copper 2 Oxide Formation.
From study.com
Copper II Oxide Formula, Properties & Structure Lesson Copper 2 Oxide Formation Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. The formation of copper oxide often results from the oxidation of copper. How do you write copper (ii) oxide? It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. As the name suggests, copper (ii) oxide. Copper 2 Oxide Formation.
From testbook.com
Copper Oxide Learn Definition, Formula, Structure & Properties Copper 2 Oxide Formation As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). If they weigh the reactants and products. It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane. Copper 2 Oxide Formation.
From www.sciencephoto.com
Copper (II) oxide Stock Image C014/6955 Science Photo Library Copper 2 Oxide Formation This process typically happens in the presence of water and oxygen in. If they weigh the reactants and products. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. The ‘ii’ in its name signifies that the copper ion. It is a black material that often coats old. Copper 2 Oxide Formation.
From www.chemtube3d.com
Copper (I) Oxide Cu2O Copper 2 Oxide Formation Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. This process typically happens in the presence of water and oxygen in. A black solid, it. Copper 2 Oxide Formation.
From www.youtube.com
How to Balance CuO + H2 = Cu + H2O Copper (II) Oxide and Hydrogen Gas Copper 2 Oxide Formation How do you write copper (ii) oxide? Copper (ii) oxide is made of. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. The ‘ii’ in its name signifies that the copper ion. It is a black material that. Copper 2 Oxide Formation.
From www.researchgate.net
Ellingham diagram of copper oxides for an O 2 molecule. Energy level Copper 2 Oxide Formation Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. The formation of copper oxide often results from the oxidation of copper. The ‘ii’ in its name signifies that the copper ion. Copper(ii) oxide or. Copper 2 Oxide Formation.
From www.alamy.com
Fully labelled diagram showing the reduction of copper (II) oxide using Copper 2 Oxide Formation The ‘ii’ in its name signifies that the copper ion. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. How do you write copper (ii) oxide? Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. If they weigh the reactants and products. A black solid, it is one of the two stable. Copper 2 Oxide Formation.
From www.slideserve.com
PPT Experiment 5 Some Reactions of Copper PowerPoint Presentation Copper 2 Oxide Formation Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Copper (ii) oxide is made of. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. The ‘ii’ in its name signifies that the copper ion. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the. Copper 2 Oxide Formation.
From www.slideserve.com
PPT Copper Functioning as an Oxygen Carrier in Chemical Looping Copper 2 Oxide Formation Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it,. Copper 2 Oxide Formation.
From pubs.acs.org
Initial Stages of Oxide Formation on Copper Surfaces during Oxygen Copper 2 Oxide Formation The formation of copper oxide often results from the oxidation of copper. How do you write copper (ii) oxide? A black solid, it is one of the two stable oxides of copper, the. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. It is a black material that often coats old pieces of copper, most commonly. Copper 2 Oxide Formation.
From www.slideserve.com
PPT Thermal Reactions PowerPoint Presentation, free Copper 2 Oxide Formation In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. This process typically happens in the presence of water and oxygen in. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper.. Copper 2 Oxide Formation.
From www.seekpng.com
Copper Ii Oxide Structure PNG Image Transparent PNG Free Download on Copper 2 Oxide Formation The formation of copper oxide often results from the oxidation of copper. It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. A black solid, it is one of the two stable oxides of copper, the. If they weigh the reactants and products. How do you write copper (ii). Copper 2 Oxide Formation.
From www.alamy.com
Copper (II) oxide (CuO). This chemical contains copper in a +2 Copper 2 Oxide Formation If they weigh the reactants and products. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. A black solid, it is one of the two stable oxides of copper, the. The formation of copper oxide often results from the oxidation of copper. This. Copper 2 Oxide Formation.
From www.researchgate.net
(a) TEM and SAED image of copper(II) oxide nanoparticles (b) HRTEM Copper 2 Oxide Formation It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. If they weigh the reactants and products. The formation of copper oxide often results from the oxidation of copper. As the name suggests, copper (ii) oxide. Copper 2 Oxide Formation.
From bnrc.springeropen.com
Copper(II) oxide nanocatalyst preparation and characterization green Copper 2 Oxide Formation It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. This process typically happens in the presence of water and oxygen in. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. If they weigh the reactants. Copper 2 Oxide Formation.
From www.slideserve.com
PPT Chemical Reactions Copper Reactions PowerPoint Presentation Copper 2 Oxide Formation The ‘ii’ in its name signifies that the copper ion. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. The formation of copper oxide often results from the oxidation of copper. Copper (ii) oxide. Copper 2 Oxide Formation.
From www.chegg.com
Solved Question 17 Consider the reaction of copper (II) Copper 2 Oxide Formation If they weigh the reactants and products. The formation of copper oxide often results from the oxidation of copper. It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Copper(ii) oxide. Copper 2 Oxide Formation.
From www.scribd.com
The Empirical Formula of Copper II Oxide PDF Oxide Oxygen Copper 2 Oxide Formation In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. Copper (ii) oxide is made of. A black solid, it is one of the two stable oxides of copper, the. The formation of copper oxide. Copper 2 Oxide Formation.
From www.biosynth.com
FC160925 1317380 Copper(II) oxide Biosynth Copper 2 Oxide Formation The ‘ii’ in its name signifies that the copper ion. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. A black solid, it is one of the two stable oxides of copper, the. As. Copper 2 Oxide Formation.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper 2 Oxide Formation Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. This process typically happens in the presence of water and oxygen in. Copper (ii) oxide is made of. It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. The ‘ii’ in its name signifies that the. Copper 2 Oxide Formation.
From www.slideshare.net
Chapter 4 Copper 2 Oxide Formation In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. The formation of copper oxide often results from the oxidation of copper. If they weigh the reactants and products. How do you write copper (ii) oxide? This process typically happens in the presence of water and oxygen in.. Copper 2 Oxide Formation.
From www.youtube.com
Heating a mixture of carbon and copper (II) oxide YouTube Copper 2 Oxide Formation It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. A black solid, it is one of the two stable oxides of copper, the. If they weigh. Copper 2 Oxide Formation.
From noahchemicals.com
4 Uses of Cupric Oxide (Copper II Oxide) Noah Chemicals Copper 2 Oxide Formation The ‘ii’ in its name signifies that the copper ion. In this experiment, students heat copper(ii) oxide in a glass tube while passing methane over it, reducing the copper(ii) oxide to copper. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. A black solid, it is one of the two stable oxides of copper, the. The. Copper 2 Oxide Formation.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Finding the formula of copper(II) oxide Experiment RSC Education Copper 2 Oxide Formation The formation of copper oxide often results from the oxidation of copper. A black solid, it is one of the two stable oxides of copper, the. Copper (ii) oxide is made of. If they weigh the reactants and products. Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. As the name suggests, copper (ii) oxide consists. Copper 2 Oxide Formation.
From atelier-yuwa.ciao.jp
Finding The Formula Of Copper(II) Oxide Experiment RSC Education Copper 2 Oxide Formation This process typically happens in the presence of water and oxygen in. It is a black material that often coats old pieces of copper, most commonly found in pure form as a powder. The formation of copper oxide often results from the oxidation of copper. The ‘ii’ in its name signifies that the copper ion. Copper (ii) oxide is made. Copper 2 Oxide Formation.
From www.youtube.com
Type of Reaction for Cu(OH)2 = CuO + H2O YouTube Copper 2 Oxide Formation The formation of copper oxide often results from the oxidation of copper. This process typically happens in the presence of water and oxygen in. The ‘ii’ in its name signifies that the copper ion. Copper(ii) oxide or cupric oxide (formula cuo) is a high oxide of copper. It is a black material that often coats old pieces of copper, most. Copper 2 Oxide Formation.
From www.youtube.com
Chemical Properties of Hydrogen Reaction of hydrogen with copper II Copper 2 Oxide Formation If they weigh the reactants and products. How do you write copper (ii) oxide? As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Copper (ii) oxide is made of. This process typically happens in the presence of water and oxygen in. A black solid, it is one of the two stable. Copper 2 Oxide Formation.
From www.researchgate.net
SEMimages of Cu(II) oxide anodically formed on polycrystalline copper Copper 2 Oxide Formation The ‘ii’ in its name signifies that the copper ion. Copper (ii) oxide is made of. The formation of copper oxide often results from the oxidation of copper. As the name suggests, copper (ii) oxide consists of one copper atom (cu) and one oxygen atom (o). Copper(ii) oxide or cupric oxide is an inorganic compound with the formula cuo. It. Copper 2 Oxide Formation.