Zinc And Hydrochloric Acid Temperature . An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The hydrogen gas rises in form of bubbles. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. Every chemical reaction either produces or absorbs heat. The reaction between hydrochloric acid and zinc. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). 5.2 oxidation of zinc by hydrochloric acid subject: Acid + metal → salt +. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic. This reaction is commonly used in classrooms to illustrate important concepts in Properties of zinc and specifics of its interactions with hcl.
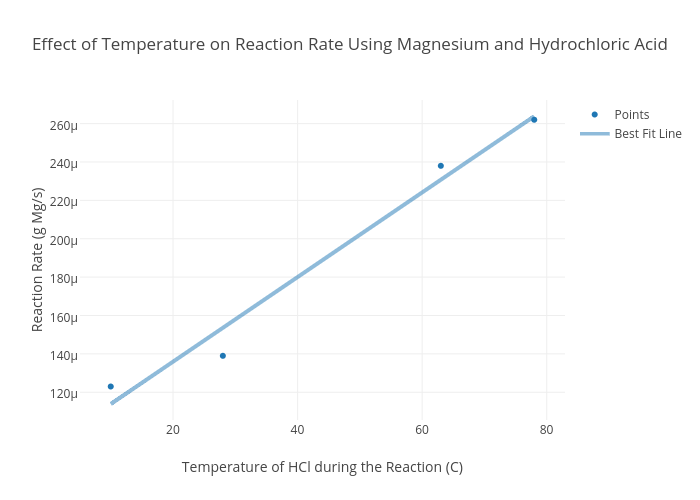
from chart-studio.plotly.com
This reaction is commonly used in classrooms to illustrate important concepts in 5.2 oxidation of zinc by hydrochloric acid subject: The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Acid + metal → salt +. The reaction between hydrochloric acid and zinc. Every chemical reaction either produces or absorbs heat. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic.
Effect of Temperature on Reaction Rate Using Magnesium and Hydrochloric
Zinc And Hydrochloric Acid Temperature The hydrogen gas rises in form of bubbles. 5.2 oxidation of zinc by hydrochloric acid subject: The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). The hydrogen gas rises in form of bubbles. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. Properties of zinc and specifics of its interactions with hcl. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic. An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). Acid + metal → salt +. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Every chemical reaction either produces or absorbs heat. The reaction between hydrochloric acid and zinc. This reaction is commonly used in classrooms to illustrate important concepts in
From www.sciencephoto.com
Zinc reacts with hydrochloric acid Stock Image C052/7641 Science Zinc And Hydrochloric Acid Temperature The reaction between hydrochloric acid and zinc. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). The hydrogen gas rises in form of bubbles. Acid + metal → salt +. 5.2. Zinc And Hydrochloric Acid Temperature.
From plotly.com
Hydrochloric AcidSodium Thiosulfate Reaction scatter chart made by Zinc And Hydrochloric Acid Temperature The hydrogen gas rises in form of bubbles. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic. 5.2 oxidation of zinc by hydrochloric acid subject: Properties of zinc and specifics of its interactions with hcl. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The metal zinc readily reacts with hydrochloric. Zinc And Hydrochloric Acid Temperature.
From www.teachoo.com
Assertion (A) When zinc is added to dilute hydrochloric acid, hydro Zinc And Hydrochloric Acid Temperature The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). Acid + metal → salt +. An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The zinc and. Zinc And Hydrochloric Acid Temperature.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid Temperature The reaction between hydrochloric acid and zinc. Acid + metal → salt +. An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The hydrogen gas rises in form of bubbles. Acids will react with reactive. Zinc And Hydrochloric Acid Temperature.
From slideplayer.com
Chemical Reactions & Equations ppt download Zinc And Hydrochloric Acid Temperature The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic. The reaction between hydrochloric acid and zinc. Acid + metal → salt. Zinc And Hydrochloric Acid Temperature.
From www.echemi.com
How is the specific gravity of hydrochloric acid determined? ECHEMI Zinc And Hydrochloric Acid Temperature Acid + metal → salt +. The reaction between hydrochloric acid and zinc. This reaction is commonly used in classrooms to illustrate important concepts in The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The hydrogen gas rises in form of bubbles. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations,. Zinc And Hydrochloric Acid Temperature.
From www.youtube.com
Zn + HCl Reaction Zinc + Hydrochloric Acid YouTube Zinc And Hydrochloric Acid Temperature Every chemical reaction either produces or absorbs heat. An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. The hydrogen gas rises in form of bubbles. The reaction between zinc metal and the. Zinc And Hydrochloric Acid Temperature.
From www.transtutors.com
(Solved) Question 1. 150.0 G Of Dilute Hydrochloric Acid Is Placed Zinc And Hydrochloric Acid Temperature 5.2 oxidation of zinc by hydrochloric acid subject: The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Acid + metal → salt +. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. Acids will react with reactive metals, such. Zinc And Hydrochloric Acid Temperature.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc And Hydrochloric Acid Temperature An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). Every chemical reaction either produces or absorbs heat. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The reaction between hydrochloric acid and zinc. 5.2 oxidation of zinc by hydrochloric acid subject: Acid + metal →. Zinc And Hydrochloric Acid Temperature.
From www.nagwa.com
Question Video Describing the Correct Symbol Equation for the Reaction Zinc And Hydrochloric Acid Temperature Properties of zinc and specifics of its interactions with hcl. Acid + metal → salt +. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Oxidation/reduction, gas forming reaction, acid properties,. Zinc And Hydrochloric Acid Temperature.
From www.coursehero.com
[Solved] TABLE 2 Temperature of hydrochloric acid 20.7 .C Temperature Zinc And Hydrochloric Acid Temperature Every chemical reaction either produces or absorbs heat. This reaction is commonly used in classrooms to illustrate important concepts in An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). Acid + metal → salt +. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction.. Zinc And Hydrochloric Acid Temperature.
From www.numerade.com
In this problem, zinc reacts with hydrochloric acid to make zinc Zinc And Hydrochloric Acid Temperature This reaction is commonly used in classrooms to illustrate important concepts in The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. Acid + metal → salt +. 5.2 oxidation of zinc by hydrochloric acid subject: The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2). Zinc And Hydrochloric Acid Temperature.
From www.youtube.com
Reaction of Zinc and Hydrochloric acid YouTube Zinc And Hydrochloric Acid Temperature Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). 5.2 oxidation of zinc by hydrochloric acid subject: The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction.. Zinc And Hydrochloric Acid Temperature.
From www.engineeringtoolbox.com
Density of Aqueous Solutions of some Substances Zinc And Hydrochloric Acid Temperature 5.2 oxidation of zinc by hydrochloric acid subject: The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The hydrogen gas rises in form of bubbles. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). Acids will react with reactive metals, such as magnesium. Zinc And Hydrochloric Acid Temperature.
From www.bartleby.com
Answered A common way to make hydrogen gas in… bartleby Zinc And Hydrochloric Acid Temperature Every chemical reaction either produces or absorbs heat. An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). Oxidation/reduction, gas. Zinc And Hydrochloric Acid Temperature.
From readingandwritingprojectcom.web.fc2.com
zinc metal and hydrochloric acid Zinc And Hydrochloric Acid Temperature Every chemical reaction either produces or absorbs heat. Acid + metal → salt +. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The metal zinc readily reacts with hydrochloric acid. Zinc And Hydrochloric Acid Temperature.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0309 Science Zinc And Hydrochloric Acid Temperature Properties of zinc and specifics of its interactions with hcl. The hydrogen gas rises in form of bubbles. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). The reaction between zinc. Zinc And Hydrochloric Acid Temperature.
From www.numerade.com
SOLVED A 20.00 g mixture of magnesium and zinc metal reacting with Zinc And Hydrochloric Acid Temperature This reaction is commonly used in classrooms to illustrate important concepts in The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. An example of this type of reaction is hydrochloric. Zinc And Hydrochloric Acid Temperature.
From www.researchgate.net
The corrosion parameters of zinc in 1.0 N Hydrochloric acid containing Zinc And Hydrochloric Acid Temperature Every chemical reaction either produces or absorbs heat. The hydrogen gas rises in form of bubbles. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. 5.2 oxidation of zinc by hydrochloric acid subject: The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts. Zinc And Hydrochloric Acid Temperature.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc And Hydrochloric Acid Temperature This reaction is commonly used in classrooms to illustrate important concepts in 5.2 oxidation of zinc by hydrochloric acid subject: The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. The hydrogen gas rises in form of bubbles. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs. Zinc And Hydrochloric Acid Temperature.
From quizlet.com
investigate reactions between dilute hydrochloric and sulfuric acids Zinc And Hydrochloric Acid Temperature Properties of zinc and specifics of its interactions with hcl. Acid + metal → salt +. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. Every chemical reaction either produces or absorbs heat. The reaction between hydrochloric acid and zinc. An example of this type of reaction is. Zinc And Hydrochloric Acid Temperature.
From warreninstitute.org
Unlock The POWER Of Chemical Reaction Zn + HCl To Zinc + Hydrochloric Acid Zinc And Hydrochloric Acid Temperature An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). The hydrogen gas rises in form of bubbles. The reaction between hydrochloric acid and zinc. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. Acid + metal → salt +. Every chemical reaction either produces. Zinc And Hydrochloric Acid Temperature.
From www.doubtnut.com
In a testtube hydrochloric acid is poured over a few zinc granul Zinc And Hydrochloric Acid Temperature An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). The reaction between hydrochloric acid and zinc. Acid + metal → salt +. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic. The zinc and hydrochloric. Zinc And Hydrochloric Acid Temperature.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0622 Zinc And Hydrochloric Acid Temperature The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). This reaction is commonly used in classrooms to illustrate important concepts in The hydrogen gas rises in form of bubbles. Acid +. Zinc And Hydrochloric Acid Temperature.
From chart-studio.plotly.com
Effect of Temperature on Reaction Rate Using Magnesium and Hydrochloric Zinc And Hydrochloric Acid Temperature Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic. Properties of zinc and specifics of its interactions with hcl. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). 5.2 oxidation of zinc by hydrochloric acid subject: Every chemical reaction either produces or absorbs heat. Acid + metal → salt +. This. Zinc And Hydrochloric Acid Temperature.
From exoxsryij.blob.core.windows.net
Zinc And Hydrochloric Acid React To Make Zinc Chloride And Hydrogen at Zinc And Hydrochloric Acid Temperature The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). 5.2 oxidation of zinc by hydrochloric acid subject: Acid + metal → salt +. The hydrogen gas rises in form of bubbles. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. An example of this. Zinc And Hydrochloric Acid Temperature.
From express.adobe.com
Zinc and Hydrochloric Acid Zinc And Hydrochloric Acid Temperature 5.2 oxidation of zinc by hydrochloric acid subject: The hydrogen gas rises in form of bubbles. Oxidation/reduction, gas forming reaction, acid properties, net ionic equations, exothermic. The reaction between hydrochloric acid and zinc. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. An example of this type of reaction is hydrochloric acid. Zinc And Hydrochloric Acid Temperature.
From www.numerade.com
SOLVED Zinc metal reacts with hydrochloric acid according to the Zinc And Hydrochloric Acid Temperature Acid + metal → salt +. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). The reaction between zinc. Zinc And Hydrochloric Acid Temperature.
From thewonderofscience.com
Reaction of zinc and hydrochloric acid (NY) — The Wonder of Science Zinc And Hydrochloric Acid Temperature 5.2 oxidation of zinc by hydrochloric acid subject: Acid + metal → salt +. Properties of zinc and specifics of its interactions with hcl. The reaction between hydrochloric acid and zinc. Every chemical reaction either produces or absorbs heat. An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). Oxidation/reduction, gas forming reaction, acid. Zinc And Hydrochloric Acid Temperature.
From www.numerade.com
SOLVED Consider the reaction between zinc metal and hydrochloric acid Zinc And Hydrochloric Acid Temperature 5.2 oxidation of zinc by hydrochloric acid subject: Acid + metal → salt +. Every chemical reaction either produces or absorbs heat. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). The reaction between hydrochloric acid and zinc. Properties of zinc and specifics of its interactions with hcl. Oxidation/reduction, gas forming reaction,. Zinc And Hydrochloric Acid Temperature.
From brainly.com
a student was investigating the reaction between marble chips and Zinc And Hydrochloric Acid Temperature The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. An example of this type of reaction is hydrochloric acid reacting with the metal zinc (zn). Every chemical reaction either produces or absorbs heat. The reaction between hydrochloric acid and zinc. The reaction between zinc metal and the hydrochloric. Zinc And Hydrochloric Acid Temperature.
From byjus.com
Zinc and hydrochloric acid react according t reaction Zn(s) + 2HCI(aq Zinc And Hydrochloric Acid Temperature 5.2 oxidation of zinc by hydrochloric acid subject: Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. This reaction is commonly used in classrooms to illustrate important concepts in The metal. Zinc And Hydrochloric Acid Temperature.
From www.sciencephoto.com
Zinc reacting with hydrochloric acid Stock Image A500/0662 Zinc And Hydrochloric Acid Temperature The reaction between hydrochloric acid and zinc. The reaction between zinc metal and the hydrochloric acid solution is an example of a single displacement reaction. This reaction is commonly used in classrooms to illustrate important concepts in Acid + metal → salt +. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen.. Zinc And Hydrochloric Acid Temperature.
From www.chegg.com
Solved 1. Zinc metal reacts with hydrochloric acid according Zinc And Hydrochloric Acid Temperature The reaction between hydrochloric acid and zinc. Properties of zinc and specifics of its interactions with hcl. The zinc and hydrochloric acid reaction is a highly fascinating chemical reaction that occurs when zinc metal reacts with hydrochloric acid. Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. Oxidation/reduction, gas forming reaction, acid. Zinc And Hydrochloric Acid Temperature.
From fphoto.photoshelter.com
science chemistry redox reaction zinc hydrochloric acid Fundamental Zinc And Hydrochloric Acid Temperature Acids will react with reactive metals, such as magnesium and zinc, to make a salt and hydrogen. The reaction between hydrochloric acid and zinc. The metal zinc readily reacts with hydrochloric acid to produce hydrogen gas (h2) and zinc chloride (zncl2). Acid + metal → salt +. The hydrogen gas rises in form of bubbles. This reaction is commonly used. Zinc And Hydrochloric Acid Temperature.