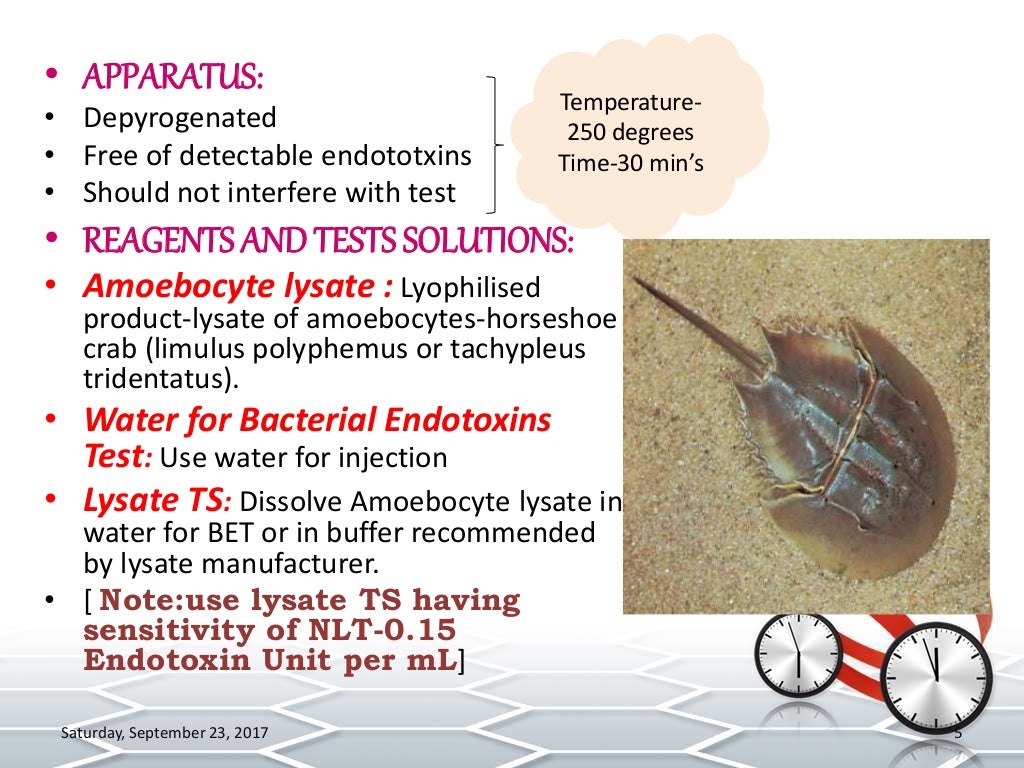
Bacterial Endotoxin Test Validation Protocol . Test preparation of solutions standard. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. The steps detailed above with an existing test method. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Bacterial endotoxin test product validation reference protocol no.: Product validation using the mcs™ entails the same steps that are followed for other lal. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: Organizations are continuously testing for endotoxins to ensure their. Endotoxin testing is a major component in product manufacturing.
from www.slideshare.net
Test preparation of solutions standard. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: Product validation using the mcs™ entails the same steps that are followed for other lal. The steps detailed above with an existing test method. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Organizations are continuously testing for endotoxins to ensure their. Endotoxin testing is a major component in product manufacturing. Bacterial endotoxin test product validation reference protocol no.:
Bacterial Endotoxins test
Bacterial Endotoxin Test Validation Protocol Organizations are continuously testing for endotoxins to ensure their. Bacterial endotoxin test product validation reference protocol no.: Organizations are continuously testing for endotoxins to ensure their. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Endotoxin testing is a major component in product manufacturing. Product validation using the mcs™ entails the same steps that are followed for other lal. The steps detailed above with an existing test method. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Test preparation of solutions standard.
From studylib.net
Bacterial Endotoxin Test by Gel Clot Method Bacterial Endotoxin Test Validation Protocol This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Bacterial endotoxin test product validation reference protocol no.: Endotoxin testing is a major component in product manufacturing. Organizations are continuously testing for endotoxins to ensure their. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉. Bacterial Endotoxin Test Validation Protocol.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test Validation Protocol Endotoxin testing is a major component in product manufacturing. Test preparation of solutions standard. Organizations are continuously testing for endotoxins to ensure their. The steps detailed above with an existing test method. Product validation using the mcs™ entails the same steps that are followed for other lal. Bacterial endotoxin test product validation reference protocol no.: Stage 6 harmonization official december. Bacterial Endotoxin Test Validation Protocol.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test Validation Protocol Bacterial endotoxin test product validation reference protocol no.: Test preparation of solutions standard. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Endotoxin testing is a major component in product manufacturing. Organizations are continuously testing for endotoxins to ensure their. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins. Bacterial Endotoxin Test Validation Protocol.
From www.pharmasopworld.com
Endotoxin testing for sterile raw materials Bacterial Endotoxin Test Validation Protocol Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: The steps detailed above with an existing test method. Bacterial endotoxin test product validation reference protocol no.: Test preparation of solutions standard. Endotoxin testing is a major component in product manufacturing. This booklet covers below chapter that is taken from pda's bestselling laboratory. Bacterial Endotoxin Test Validation Protocol.
From www.drawittoknowit.com
Immunology/Microbiology Glossary Bacteria Endotoxin Draw It to Know It Bacterial Endotoxin Test Validation Protocol Organizations are continuously testing for endotoxins to ensure their. Test preparation of solutions standard. Product validation using the mcs™ entails the same steps that are followed for other lal. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan. Bacterial Endotoxin Test Validation Protocol.
From www.youtube.com
BET BACTERIAL ENDOTOXIN TEST YouTube Bacterial Endotoxin Test Validation Protocol Test preparation of solutions standard. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Organizations are continuously testing for endotoxins to ensure their. Endotoxin testing is a major. Bacterial Endotoxin Test Validation Protocol.
From www.youtube.com
USP CHAPTER 1085, “GUIDELINES ON THE ENDOTOXINS TEST” YouTube Bacterial Endotoxin Test Validation Protocol Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: The steps detailed above with an existing test method. Product validation using the mcs™ entails the same steps that are followed for other lal. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor.. Bacterial Endotoxin Test Validation Protocol.
From zhuanlan.zhihu.com
Bacterial Endotix Testing (BET) 细菌内毒素测试 知乎 Bacterial Endotoxin Test Validation Protocol Test preparation of solutions standard. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Endotoxin testing is a major component in product manufacturing. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Bacterial endotoxin test product validation. Bacterial Endotoxin Test Validation Protocol.
From www.slideshare.net
Bacterial Endotoxins test Bacterial Endotoxin Test Validation Protocol Endotoxin testing is a major component in product manufacturing. The steps detailed above with an existing test method. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Bacterial endotoxin test. Bacterial Endotoxin Test Validation Protocol.
From ethidelabs.com
Ethide Laboratories Bacterial Endotoxin Testing Bacterial Endotoxin Test Validation Protocol Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. The steps detailed above with an existing test method. Bacterial endotoxin test product validation reference protocol no.: Organizations are continuously testing for endotoxins to ensure their. Endotoxin testing is a major component in product manufacturing. This booklet covers below chapter that. Bacterial Endotoxin Test Validation Protocol.
From siglaboratory.com
Endotoxin Test SIG LABORATORY Bacterial Endotoxin Test Validation Protocol The steps detailed above with an existing test method. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Bacterial endotoxin test product validation reference protocol no.: Organizations are continuously testing for endotoxins to ensure their. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change. Bacterial Endotoxin Test Validation Protocol.
From www.shutterstock.com
23 Bacterial endotoxin 图片、库存照片和矢量图 Shutterstock Bacterial Endotoxin Test Validation Protocol This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: Product validation using the mcs™ entails the same steps that are followed for other lal. Bacterial endotoxin test product validation reference. Bacterial Endotoxin Test Validation Protocol.
From www.mdpi.com
Toxins Free FullText Endotoxins from a Pharmacopoeial Point of View Bacterial Endotoxin Test Validation Protocol Test preparation of solutions standard. The steps detailed above with an existing test method. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Bacterial endotoxin test product validation reference protocol no.: Organizations are continuously testing for endotoxins to ensure their. Product validation using the mcs™ entails the same steps that. Bacterial Endotoxin Test Validation Protocol.
From microbeonline.com
Pyrogen and Bacterial Endotoxin Testing Methods • Microbe Online Bacterial Endotoxin Test Validation Protocol Organizations are continuously testing for endotoxins to ensure their. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins. Bacterial Endotoxin Test Validation Protocol.
From www.biomerieux-industry.com
BACTERIAL ENDOTOXIN TESTING 10 reasons to choose factor C Bacterial Endotoxin Test Validation Protocol This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: Test preparation. Bacterial Endotoxin Test Validation Protocol.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test Validation Protocol The steps detailed above with an existing test method. Bacterial endotoxin test product validation reference protocol no.: Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Organizations are continuously testing for endotoxins to ensure their. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers. Bacterial Endotoxin Test Validation Protocol.
From pharmajia.com
SOP for Bacterial endotoxin test by gel clot method PharmaJia Bacterial Endotoxin Test Validation Protocol Bacterial endotoxin test product validation reference protocol no.: This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36,. Bacterial Endotoxin Test Validation Protocol.
From www.researchgate.net
(PDF) Validation of bacterial endotoxin test for injectable batch Bacterial Endotoxin Test Validation Protocol The steps detailed above with an existing test method. Product validation using the mcs™ entails the same steps that are followed for other lal. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan. Bacterial Endotoxin Test Validation Protocol.
From eagleanalytical.com
Bacterial Endotoxin Test USP 85 Eagle Analytical Bacterial Endotoxin Test Validation Protocol Endotoxin testing is a major component in product manufacturing. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Organizations are continuously testing for endotoxins to ensure their. Bacterial endotoxin test product validation reference protocol no.: Product validation using the mcs™ entails the same steps that are followed for other lal.. Bacterial Endotoxin Test Validation Protocol.
From www.bioendo.com
Professional New Fashion Design for Bacterial Endotoxin Test Method Bacterial Endotoxin Test Validation Protocol Endotoxin testing is a major component in product manufacturing. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot. Bacterial Endotoxin Test Validation Protocol.
From www.pinterest.co.uk
endotoxin vs exotoxin in 2023 Lipopolysaccharide, Microbiology Bacterial Endotoxin Test Validation Protocol Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: Endotoxin testing is a major component in product manufacturing. Organizations are continuously testing for endotoxins to ensure their. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. This booklet covers below chapter that. Bacterial Endotoxin Test Validation Protocol.
From www.europeanpharmaceuticalreview.com
ISO publishes standard on testing bacterial endotoxins Bacterial Endotoxin Test Validation Protocol The steps detailed above with an existing test method. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: Endotoxin testing is a major component in product manufacturing. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Test preparation of. Bacterial Endotoxin Test Validation Protocol.
From www.genobio-pharm.com
CE Certification Bacteria Endotoxin Detection Kit (Chromogenic Method Bacterial Endotoxin Test Validation Protocol Test preparation of solutions standard. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: The steps detailed above with an existing test method. Bacterial endotoxin test product validation reference protocol. Bacterial Endotoxin Test Validation Protocol.
From ethidelabs.com
Ethide Laboratories Bacterial Endotoxin Testing For Medical Devices Bacterial Endotoxin Test Validation Protocol Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Product validation using the mcs™ entails the same steps that are followed for other lal. Endotoxin testing is a major component in product manufacturing. The steps detailed above with an existing test method. This booklet covers below chapter that is taken. Bacterial Endotoxin Test Validation Protocol.
From www.slideshare.net
Bacterial Endotoxins test Bacterial Endotoxin Test Validation Protocol Bacterial endotoxin test product validation reference protocol no.: Endotoxin testing is a major component in product manufacturing. Organizations are continuously testing for endotoxins to ensure their. Test preparation of solutions standard. Product validation using the mcs™ entails the same steps that are followed for other lal. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan. Bacterial Endotoxin Test Validation Protocol.
From www.ethidelabs.com
Ethide Laboratories Bacterial Endotoxin Testing Bacterial Endotoxin Test Validation Protocol Test preparation of solutions standard. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: The steps detailed above with an existing test method. Endotoxin testing is a major component in product manufacturing. Bacterial endotoxin test product validation reference protocol no.: Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot. Bacterial Endotoxin Test Validation Protocol.
From www.americanpharmaceuticalreview.com
Method Changes for Bacterial Endotoxins Testing (BET) Steps to Follow Bacterial Endotoxin Test Validation Protocol The steps detailed above with an existing test method. Endotoxin testing is a major component in product manufacturing. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Organizations. Bacterial Endotoxin Test Validation Protocol.
From www.scribd.com
bacterial endotoxin test Bacterial Endotoxin Test Validation Protocol Bacterial endotoxin test product validation reference protocol no.: This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: Organizations are continuously testing for endotoxins to ensure their. Product validation using the. Bacterial Endotoxin Test Validation Protocol.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test Validation Protocol Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Bacterial endotoxin test product validation reference protocol no.: Product validation using the mcs™ entails the same steps that are followed for other lal. Test preparation of solutions standard. Organizations are continuously testing for endotoxins to ensure their. The steps detailed above. Bacterial Endotoxin Test Validation Protocol.
From microbiologyguideritesh.com
Bacterial Endotoxin Test Bacterial Endotoxin Test Validation Protocol Endotoxin testing is a major component in product manufacturing. Test preparation of solutions standard. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Bacterial endotoxin test product validation reference protocol no.: Product validation using the mcs™ entails the same steps that are followed for other lal. Stage 6 harmonization official. Bacterial Endotoxin Test Validation Protocol.
From njlabs.com
Endotoxin Testing Services Endotoxin Analysis Lab NJ Labs Bacterial Endotoxin Test Validation Protocol Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Test preparation of solutions standard. This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Endotoxin testing is a major component in product manufacturing. Organizations are continuously testing for. Bacterial Endotoxin Test Validation Protocol.
From www.slideshare.net
Bacterial Endotoxins test Bacterial Endotoxin Test Validation Protocol This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Test preparation of solutions standard. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins. Bacterial Endotoxin Test Validation Protocol.
From www.nelsonlabs.com
Bacterial Endotoxins Test (BET) Services Nelson Labs Bacterial Endotoxin Test Validation Protocol Product validation using the mcs™ entails the same steps that are followed for other lal. Endotoxin testing is a major component in product manufacturing. Stage 6 harmonization official december 1, 2012 〈85〉 bacterial endotoxins test1 〈85〉 bacterial endotoxins change to read: Bacterial endotoxin test product validation reference protocol no.: Organizations are continuously testing for endotoxins to ensure their. Bacterial endotoxin. Bacterial Endotoxin Test Validation Protocol.
From www.bioendo.com
Professional Bacterial Endotoxin Test Method Validation Protocol Bacterial Endotoxin Test Validation Protocol Organizations are continuously testing for endotoxins to ensure their. Bacterial endotoxin test product validation reference protocol no.: Product validation using the mcs™ entails the same steps that are followed for other lal. Endotoxin testing is a major component in product manufacturing. The steps detailed above with an existing test method. Test preparation of solutions standard. This booklet covers below chapter. Bacterial Endotoxin Test Validation Protocol.
From mycoscience.com
MycoScience Bacterial Endotoxin Testing Bacterial Endotoxin Test Validation Protocol This booklet covers below chapter that is taken from pda's bestselling laboratory validation, and offers a complete overview of a specific topic. Bacterial endotoxin test centre for quality control national pharmaceutical control bureau lot 36, jalan universiti, 46200 petaling jaya, selangor. Organizations are continuously testing for endotoxins to ensure their. Test preparation of solutions standard. Stage 6 harmonization official december. Bacterial Endotoxin Test Validation Protocol.