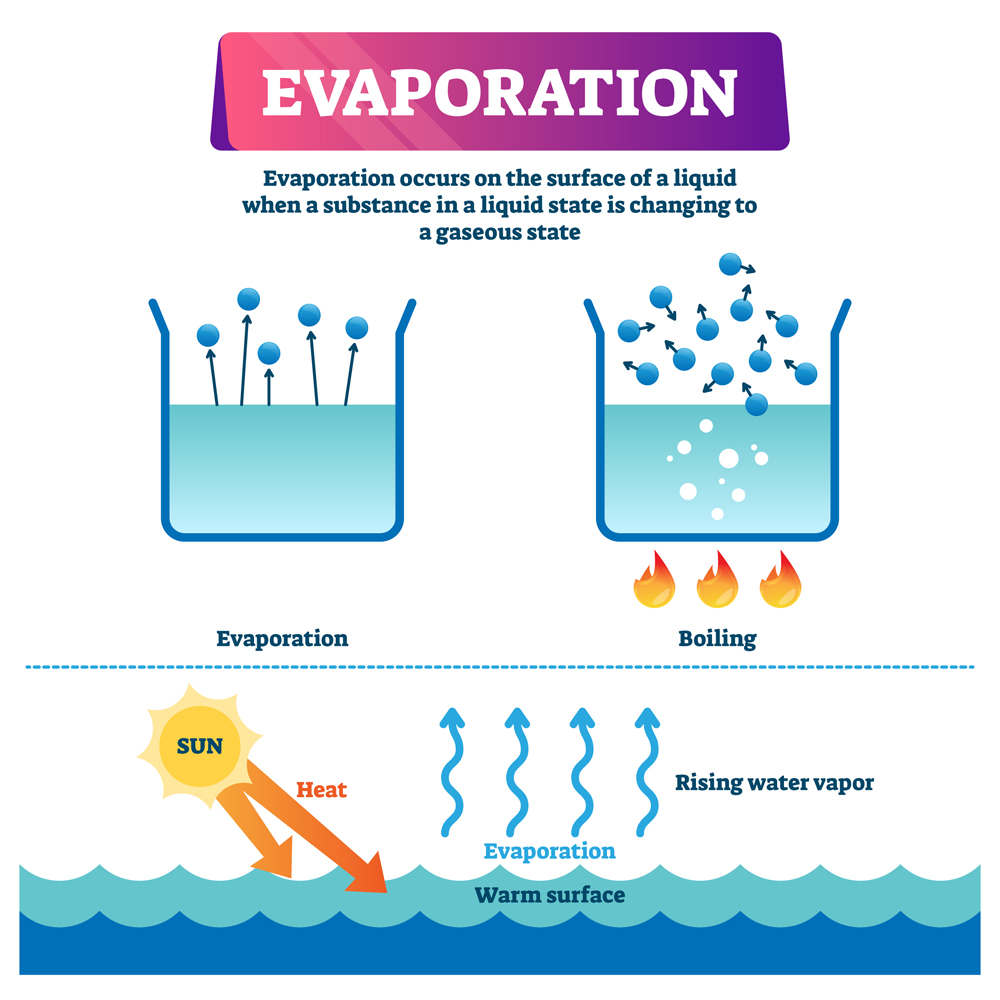
Water Evaporate At All Temperatures . However, we find that even at room temperature, water can evaporate into gas. Puddles do not boil because. Water molecules evaporate off the surface until the amount of water in the air. The boiling point of water is $\pu{100^\circ c}$. Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360°c. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. The temperature at which water in liquid form is converted into gaseous form. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into the. Puddles dry up because of evaporation. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. When liquid water meets dry air, it is not in equilibrium; Then how it possible for water to evaporate at room temperature? Boiling point of water is 100 degree celsius. Evaporation occurs at all temperatures, boiling happens at a fixed temperature depending on the liquid.
from www.scienceabc.com
Boiling point of water is 100 degree celsius. Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360°c. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Evaporation occurs at all temperatures, boiling happens at a fixed temperature depending on the liquid. Puddles do not boil because. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into the. The boiling point of water is $\pu{100^\circ c}$. When liquid water meets dry air, it is not in equilibrium; Therefore, water vapor can exist at temperatures of, say,.
Why Does Water Evaporate At Room Temperature?
Water Evaporate At All Temperatures Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360°c. Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360°c. Water molecules evaporate off the surface until the amount of water in the air. The boiling point of water is $\pu{100^\circ c}$. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Therefore, water vapor can exist at temperatures of, say,. Evaporation occurs at all temperatures, boiling happens at a fixed temperature depending on the liquid. Boiling point of water is 100 degree celsius. Puddles do not boil because. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into the. When liquid water meets dry air, it is not in equilibrium; The temperature at which water in liquid form is converted into gaseous form. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Then how it possible for water to evaporate at room temperature? Puddles dry up because of evaporation. However, we find that even at room temperature, water can evaporate into gas.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Water Evaporate At All Temperatures Therefore, water vapor can exist at temperatures of, say,. Puddles dry up because of evaporation. Then how it possible for water to evaporate at room temperature? When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. The temperature at which water in liquid form is converted into gaseous form. Liquid water is made. Water Evaporate At All Temperatures.
From 9to5science.com
[Solved] Why does water evaporate at room temperature? 9to5Science Water Evaporate At All Temperatures Therefore, water vapor can exist at temperatures of, say,. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into the. Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360°c. Water evaporates at room. Water Evaporate At All Temperatures.
From tipseri.com
How long does it take for water to evaporate at room temperature? Tipseri Water Evaporate At All Temperatures Evaporation occurs at all temperatures, boiling happens at a fixed temperature depending on the liquid. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into the. Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0. Water Evaporate At All Temperatures.
From slideplayer.com
Liquids & Vapor Pressure ppt download Water Evaporate At All Temperatures The boiling point of water is $\pu{100^\circ c}$. Puddles do not boil because. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. The temperature at. Water Evaporate At All Temperatures.
From guides.hostos.cuny.edu
Chapter 3 Solids and Liquids CHE 110 Introduction to Chemistry Water Evaporate At All Temperatures Then how it possible for water to evaporate at room temperature? The boiling point of water is $\pu{100^\circ c}$. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Water molecules evaporate off the surface until the amount of water in the air. Puddles do not boil because.. Water Evaporate At All Temperatures.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Water Evaporate At All Temperatures The boiling point of water is $\pu{100^\circ c}$. However, we find that even at room temperature, water can evaporate into gas. Boiling point of water is 100 degree celsius. When liquid water meets dry air, it is not in equilibrium; Then how it possible for water to evaporate at room temperature? Water molecules evaporate off the surface until the amount. Water Evaporate At All Temperatures.
From learningschooltrkesp5v.z22.web.core.windows.net
Water Evaporation Rate By Temperature Water Evaporate At All Temperatures Boiling point of water is 100 degree celsius. Puddles dry up because of evaporation. However, we find that even at room temperature, water can evaporate into gas. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. When the surface is exposed to sunlight, some molecules gain enough. Water Evaporate At All Temperatures.
From www.scienceabc.com
Why Does Water Evaporate At Room Temperature? Water Evaporate At All Temperatures When liquid water meets dry air, it is not in equilibrium; When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Evaporation occurs at all temperatures, boiling happens at a fixed temperature depending on the liquid. However, we find that even at room temperature, water can evaporate into gas. Boiling point of water. Water Evaporate At All Temperatures.
From www.researchgate.net
Evolution of water vapor pressure Pvapor along with interfacial Water Evaporate At All Temperatures Water molecules evaporate off the surface until the amount of water in the air. Evaporation occurs at all temperatures, boiling happens at a fixed temperature depending on the liquid. Puddles do not boil because. The boiling point of water is $\pu{100^\circ c}$. When liquid water meets dry air, it is not in equilibrium; Definitions, online calculator and figures and tables. Water Evaporate At All Temperatures.
From exyukbtsg.blob.core.windows.net
Is Boiling Water Evaporation at Lawrence Edwards blog Water Evaporate At All Temperatures When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. When liquid water meets dry air, it is not in equilibrium; If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into the. Water evaporates at room temperature because the molecules at the surface of. Water Evaporate At All Temperatures.
From watermarkexplorer.blogspot.com
Evaporation Water Evaporation Temperature Water Evaporate At All Temperatures The temperature at which water in liquid form is converted into gaseous form. Evaporation occurs at all temperatures, boiling happens at a fixed temperature depending on the liquid. When liquid water meets dry air, it is not in equilibrium; The boiling point of water is $\pu{100^\circ c}$. When the surface is exposed to sunlight, some molecules gain enough energy to. Water Evaporate At All Temperatures.
From vt.audubon.org
The Water Cycle Revisited! Audubon Vermont Water Evaporate At All Temperatures The temperature at which water in liquid form is converted into gaseous form. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. The boiling point of water is $\pu{100^\circ c}$. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere.. Water Evaporate At All Temperatures.
From www.numerade.com
SOLVED The rate at which water evaporates from a certain reservoir Water Evaporate At All Temperatures However, we find that even at room temperature, water can evaporate into gas. Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360°c. When liquid water meets dry air, it is not in equilibrium; Boiling point of water is 100 degree celsius. Therefore,. Water Evaporate At All Temperatures.
From www.alamy.com
Water cycle in nature environment. The sun, which drives the water Water Evaporate At All Temperatures Water molecules evaporate off the surface until the amount of water in the air. Puddles do not boil because. Boiling point of water is 100 degree celsius. Puddles dry up because of evaporation. The boiling point of water is $\pu{100^\circ c}$. Then how it possible for water to evaporate at room temperature? Water evaporates at room temperature because the molecules. Water Evaporate At All Temperatures.
From www.teachoo.com
Why is Water liquid at room temperature? Give reasons Teachoo Water Evaporate At All Temperatures When liquid water meets dry air, it is not in equilibrium; Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Then how it possible for water to evaporate at room temperature? However, we find that even at room temperature, water can evaporate into gas. Definitions, online calculator. Water Evaporate At All Temperatures.
From physics.stackexchange.com
thermodynamics Evaporation of water at room temperature Physics Water Evaporate At All Temperatures The temperature at which water in liquid form is converted into gaseous form. Water molecules evaporate off the surface until the amount of water in the air. When the surface is exposed to sunlight, some molecules gain enough energy to escape into the atmosphere. Then how it possible for water to evaporate at room temperature? However, we find that even. Water Evaporate At All Temperatures.
From www.pxfuel.com
Can Water Evaporate at all Temperatures? Are Evaporation and Boiling Water Evaporate At All Temperatures Boiling point of water is 100 degree celsius. Evaporation occurs at all temperatures, boiling happens at a fixed temperature depending on the liquid. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Puddles dry up because of evaporation. Then how it possible for water to evaporate at. Water Evaporate At All Temperatures.
From loetlngwe.blob.core.windows.net
What Temperature Does Everclear Evaporate at Jay Molter blog Water Evaporate At All Temperatures However, we find that even at room temperature, water can evaporate into gas. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into the. Water molecules evaporate off the. Water Evaporate At All Temperatures.
From slideplayer.com
Topic Vapor Pressure. ppt download Water Evaporate At All Temperatures Water molecules evaporate off the surface until the amount of water in the air. The temperature at which water in liquid form is converted into gaseous form. However, we find that even at room temperature, water can evaporate into gas. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as. Water Evaporate At All Temperatures.
From www.bol.com
Why Does Water Evaporate? All about Heat and Temperature (ebook), Rob Water Evaporate At All Temperatures Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. The boiling point of water is $\pu{100^\circ c}$. Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360°c. Evaporation occurs at. Water Evaporate At All Temperatures.
From www.worldatlas.com
The Water Cycle WorldAtlas Water Evaporate At All Temperatures Water molecules evaporate off the surface until the amount of water in the air. Therefore, water vapor can exist at temperatures of, say,. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. If water evaporates at room temperature because a small percentage of the molecules have enough. Water Evaporate At All Temperatures.
From 9to5science.com
[Solved] Does the temperature at which water evaporates 9to5Science Water Evaporate At All Temperatures Puddles do not boil because. When liquid water meets dry air, it is not in equilibrium; Boiling point of water is 100 degree celsius. Therefore, water vapor can exist at temperatures of, say,. The boiling point of water is $\pu{100^\circ c}$. Water molecules evaporate off the surface until the amount of water in the air. Evaporation occurs at all temperatures,. Water Evaporate At All Temperatures.
From bceweb.org
Saturation Vapor Pressure Chart A Visual Reference of Charts Chart Water Evaporate At All Temperatures Puddles dry up because of evaporation. The boiling point of water is $\pu{100^\circ c}$. Evaporation occurs at all temperatures, boiling happens at a fixed temperature depending on the liquid. Water molecules evaporate off the surface until the amount of water in the air. If water evaporates at room temperature because a small percentage of the molecules have enough energy to. Water Evaporate At All Temperatures.
From www.youtube.com
How does water evaporate at room temperaturevaporization and Water Evaporate At All Temperatures Evaporation occurs at all temperatures, boiling happens at a fixed temperature depending on the liquid. Therefore, water vapor can exist at temperatures of, say,. When liquid water meets dry air, it is not in equilibrium; Puddles dry up because of evaporation. Then how it possible for water to evaporate at room temperature? Liquid water is made up of molecules of. Water Evaporate At All Temperatures.
From www.scienceabc.com
Are Evaporation And Boiling The Same? » ScienceABC Water Evaporate At All Temperatures The boiling point of water is $\pu{100^\circ c}$. When liquid water meets dry air, it is not in equilibrium; Therefore, water vapor can exist at temperatures of, say,. Puddles dry up because of evaporation. If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into the. Puddles do not boil because. However,. Water Evaporate At All Temperatures.
From slideplayer.com
Chemistry of Water Chapter ppt download Water Evaporate At All Temperatures If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into the. Then how it possible for water to evaporate at room temperature? Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Therefore, water vapor can exist at temperatures. Water Evaporate At All Temperatures.
From www.watercubedesign.it
Evaporation Watercube Design Water Evaporate At All Temperatures The boiling point of water is $\pu{100^\circ c}$. The temperature at which water in liquid form is converted into gaseous form. Therefore, water vapor can exist at temperatures of, say,. Puddles do not boil because. Boiling point of water is 100 degree celsius. However, we find that even at room temperature, water can evaporate into gas. Water evaporates at room. Water Evaporate At All Temperatures.
From www.metoffice.gov.uk
The water cycle Met Office Water Evaporate At All Temperatures However, we find that even at room temperature, water can evaporate into gas. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces known as ‘hydrogen bonds’. Water molecules evaporate off the surface until the amount of water in the air. If water evaporates at room temperature because a small percentage of. Water Evaporate At All Temperatures.
From nursehub.com
Evaporation NurseHub Water Evaporate At All Temperatures Evaporation occurs at all temperatures, boiling happens at a fixed temperature depending on the liquid. The boiling point of water is $\pu{100^\circ c}$. Puddles do not boil because. Puddles dry up because of evaporation. Then how it possible for water to evaporate at room temperature? Definitions, online calculator and figures and tables with water properties like density, specific weight and. Water Evaporate At All Temperatures.
From www.youtube.com
Why Does Water Evaporate at Room Temperature? YouTube Water Evaporate At All Temperatures Puddles do not boil because. Then how it possible for water to evaporate at room temperature? Water molecules evaporate off the surface until the amount of water in the air. The boiling point of water is $\pu{100^\circ c}$. Therefore, water vapor can exist at temperatures of, say,. Boiling point of water is 100 degree celsius. However, we find that even. Water Evaporate At All Temperatures.
From stock.adobe.com
Phase change transition diagram. States matter schema. Evaporation Water Evaporate At All Temperatures If water evaporates at room temperature because a small percentage of the molecules have enough energy to escape into the. However, we find that even at room temperature, water can evaporate into gas. Therefore, water vapor can exist at temperatures of, say,. Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient. Water Evaporate At All Temperatures.
From philschatz.com
Phase Change and Latent Heat · Physics Water Evaporate At All Temperatures When liquid water meets dry air, it is not in equilibrium; Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360°c. The boiling point of water is $\pu{100^\circ c}$. The temperature at which water in liquid form is converted into gaseous form. Water. Water Evaporate At All Temperatures.
From slideplayer.com
Water. ppt download Water Evaporate At All Temperatures Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Puddles dry up because of evaporation. Water molecules evaporate off the surface until the amount of water in the air. Liquid water is made up of molecules of h 2 o attracted to one another by intermolecular forces. Water Evaporate At All Temperatures.
From loetlngwe.blob.core.windows.net
What Temperature Does Everclear Evaporate at Jay Molter blog Water Evaporate At All Temperatures Definitions, online calculator and figures and tables with water properties like density, specific weight and thermal expansion coefficient of liquid water at temperatures ranging 0 to 360°c. Puddles do not boil because. Water evaporates at room temperature because the molecules at the surface of the liquid have weaker attraction than those in the bulk. Therefore, water vapor can exist at. Water Evaporate At All Temperatures.
From mavink.com
Water Evaporation Chart Water Evaporate At All Temperatures Therefore, water vapor can exist at temperatures of, say,. The temperature at which water in liquid form is converted into gaseous form. Water molecules evaporate off the surface until the amount of water in the air. The boiling point of water is $\pu{100^\circ c}$. Liquid water is made up of molecules of h 2 o attracted to one another by. Water Evaporate At All Temperatures.