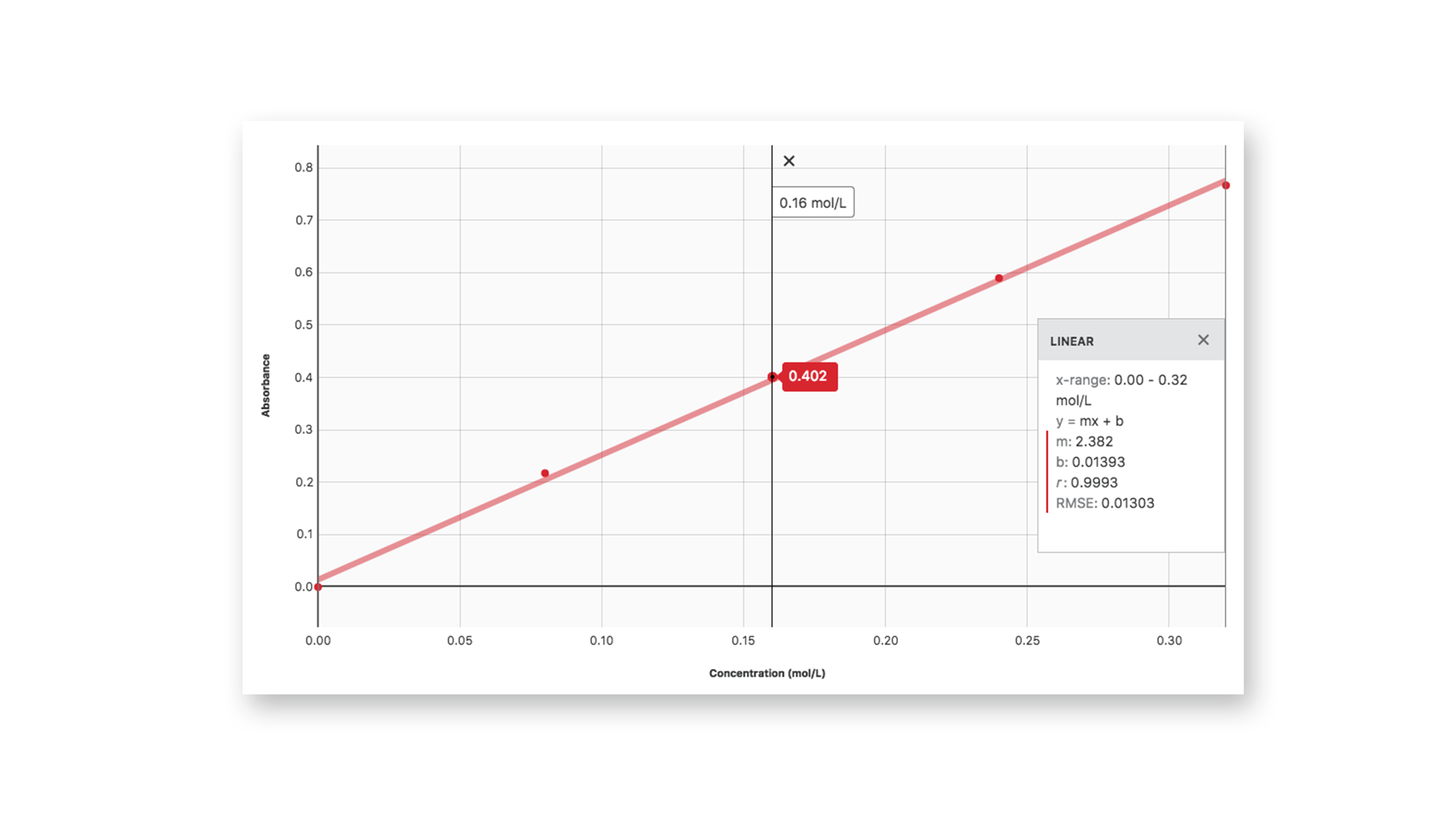
Vernier Beer's Law Lab . Calculate a standard curve from the test results of the standard solutions. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! To use spectroscopy to prepare a beer’s law plot of. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Determining the concentration of a species using a vernier spectrometer. In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate solutions. Use the beer's law plot to determine the concentration of an unknown analyte. Determine the order of reaction by measuring the absorbance of a reactant at a. The experiment in the book includes student instructions as well as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. This experiment is #11 of investigating chemistry through inquiry.
from www.vernier.com
To use spectroscopy to prepare a beer’s law plot of. Use the beer's law plot to determine the concentration of an unknown analyte. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! Determine the order of reaction by measuring the absorbance of a reactant at a. Determining the concentration of a species using a vernier spectrometer. The experiment in the book includes student instructions as well as. In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate solutions. The concentration of an unknown niso4. This experiment is #11 of investigating chemistry through inquiry. Calculate a standard curve from the test results of the standard solutions.
Go Direct® Colorimeter Vernier
Vernier Beer's Law Lab In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate solutions. In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate solutions. To use spectroscopy to prepare a beer’s law plot of. Determine the order of reaction by measuring the absorbance of a reactant at a. The experiment in the book includes student instructions as well as. Use the beer's law plot to determine the concentration of an unknown analyte. Determining the concentration of a species using a vernier spectrometer. This experiment is #11 of investigating chemistry through inquiry. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Calculate a standard curve from the test results of the standard solutions. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer!
From www.vernier.com
Go Direct® SpectroVis® Plus Spectrophotometer Vernier Vernier Beer's Law Lab To use spectroscopy to prepare a beer’s law plot of. Calculate a standard curve from the test results of the standard solutions. Determine the order of reaction by measuring the absorbance of a reactant at a. Determining the concentration of a species using a vernier spectrometer. Use the beer's law plot to determine the concentration of an unknown analyte. In. Vernier Beer's Law Lab.
From www.studocu.com
Lab report Experiment 2. Spectroscopic Analysis using Beer’s Law. Experiment 2. Name Vernier Beer's Law Lab Calculate a standard curve from the test results of the standard solutions. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Use the beer's law plot to determine the concentration of an unknown analyte. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer!. Vernier Beer's Law Lab.
From www.vernier.com
Three Ways to Enliven Remote Learning with Vernier Graphical Analysis Pro Vernier Vernier Beer's Law Lab Use the beer's law plot to determine the concentration of an unknown analyte. Calculate a standard curve from the test results of the standard solutions. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate. Vernier Beer's Law Lab.
From www.slideshare.net
How to use Vernier Caliper and how to take measurement from it. Vernier Beer's Law Lab This experiment is #11 of investigating chemistry through inquiry. The experiment in the book includes student instructions as well as. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Use the beer's law plot to determine the concentration of an unknown analyte. The direct relationship between absorbance and concentration for a solution is. Vernier Beer's Law Lab.
From www.vernier.com
Beer's Law Investigations > Experiment 11 from Investigating Chemistry through Inquiry Vernier Beer's Law Lab Calculate a standard curve from the test results of the standard solutions. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! Determine the order of reaction by measuring the absorbance of a reactant at. Vernier Beer's Law Lab.
From www.youtube.com
Spectrophotometer Beer's Law And Colorimetry Lab YouTube Vernier Beer's Law Lab The direct relationship between absorbance and concentration for a solution is known as beer’s law. The experiment in the book includes student instructions as well as. Use the beer's law plot to determine the concentration of an unknown analyte. In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate solutions. In most experiments, molar. Vernier Beer's Law Lab.
From www.studocu.com
Beer's Law Lab Beer's Law, 20192020 academic year Experiment 4 Beer’s Law. Purpose The Vernier Beer's Law Lab Use the beer's law plot to determine the concentration of an unknown analyte. The experiment in the book includes student instructions as well as. The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. This experiment is #11 of investigating chemistry through inquiry. Make colorful concentrated and dilute solutions. Vernier Beer's Law Lab.
From goformative.com
Beer's Law Simulation for AP Chemistry Carson Dobrin Library Formative Vernier Beer's Law Lab The direct relationship between absorbance and concentration for a solution is known as beer’s law. This experiment is #11 of investigating chemistry through inquiry. Use the beer's law plot to determine the concentration of an unknown analyte. The experiment in the book includes student instructions as well as. Calculate a standard curve from the test results of the standard solutions.. Vernier Beer's Law Lab.
From github.com
GitHub phetsims/beerslawlab "Beer's Law Lab" is an educational simulation in HTML5, by PhET Vernier Beer's Law Lab The experiment in the book includes student instructions as well as. The concentration of an unknown niso4. Use the beer's law plot to determine the concentration of an unknown analyte. In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate solutions. To use spectroscopy to prepare a beer’s law plot of. This experiment is. Vernier Beer's Law Lab.
From www.softpedia.com
Download Beer's Law Lab Vernier Beer's Law Lab Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! To use spectroscopy to prepare a beer’s law plot of. Use the beer's law plot to determine the concentration of an unknown analyte. The direct relationship between absorbance and concentration for a solution is known as beer’s law. In most experiments,. Vernier Beer's Law Lab.
From www.youtube.com
Beer's Law Lab Procedure YouTube Vernier Beer's Law Lab The direct relationship between absorbance and concentration for a solution is known as beer’s law. Use the beer's law plot to determine the concentration of an unknown analyte. The concentration of an unknown niso4. The experiment in the book includes student instructions as well as. Calculate a standard curve from the test results of the standard solutions. To use spectroscopy. Vernier Beer's Law Lab.
From www.vernier.com
Additivity of Heats of Reaction Hess's Law > Experiment 18 from Chemistry with Vernier Vernier Beer's Law Lab The direct relationship between absorbance and concentration for a solution is known as beer’s law. Calculate a standard curve from the test results of the standard solutions. Use the beer's law plot to determine the concentration of an unknown analyte. To use spectroscopy to prepare a beer’s law plot of. Make colorful concentrated and dilute solutions and explore how much. Vernier Beer's Law Lab.
From studylib.net
Beer`s Law Lab Vernier Beer's Law Lab Calculate a standard curve from the test results of the standard solutions. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Determining the concentration of a species using a vernier spectrometer. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! In this experiment,. Vernier Beer's Law Lab.
From www.vernier.com
Colorimeter Vernier Vernier Beer's Law Lab Determining the concentration of a species using a vernier spectrometer. The direct relationship between absorbance and concentration for a solution is known as beer’s law. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Use the beer's law plot to determine the concentration of an unknown analyte. The experiment in the book includes. Vernier Beer's Law Lab.
From www.softpedia.com
Download Beer's Law Lab Vernier Beer's Law Lab Use the beer's law plot to determine the concentration of an unknown analyte. Determining the concentration of a species using a vernier spectrometer. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! This experiment is #11 of investigating chemistry through inquiry. In most experiments, molar absorptivity (ε) and the length. Vernier Beer's Law Lab.
From www.softpedia.com
Download Beer's Law Lab 1.02 Vernier Beer's Law Lab The experiment in the book includes student instructions as well as. In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate solutions. Determine the order of reaction by measuring the absorbance of a reactant at a. To use spectroscopy to prepare a beer’s law plot of. Determining the concentration of a species using a. Vernier Beer's Law Lab.
From www.softpedia.com
Download Beer's Law Lab 1.02 Vernier Beer's Law Lab Use the beer's law plot to determine the concentration of an unknown analyte. This experiment is #11 of investigating chemistry through inquiry. Determining the concentration of a species using a vernier spectrometer. In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate solutions. The concentration of an unknown niso4. The direct relationship between absorbance. Vernier Beer's Law Lab.
From www.vernier.com
Go Direct® Colorimeter Vernier Vernier Beer's Law Lab This experiment is #11 of investigating chemistry through inquiry. Use the beer's law plot to determine the concentration of an unknown analyte. The direct relationship between absorbance and concentration for a solution is known as beer’s law. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In this experiment, you will prepare and. Vernier Beer's Law Lab.
From www.vernier.com
Determining the Concentration of a Solution Beer's Law > Experiment 17 from Advanced Chemistry Vernier Beer's Law Lab Determining the concentration of a species using a vernier spectrometer. To use spectroscopy to prepare a beer’s law plot of. Use the beer's law plot to determine the concentration of an unknown analyte. Determine the order of reaction by measuring the absorbance of a reactant at a. Calculate a standard curve from the test results of the standard solutions. In. Vernier Beer's Law Lab.
From www.youtube.com
Experiment 13 Beer's Law YouTube Vernier Beer's Law Lab Calculate a standard curve from the test results of the standard solutions. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! Use the beer's law plot to determine the concentration of an unknown analyte. To. Vernier Beer's Law Lab.
From www.youtube.com
Lt Chemistry Collection, developed in partnership with Vernier Beer's Law Lab Walkthrough Vernier Beer's Law Lab In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. To use spectroscopy to prepare a beer’s law plot of. Calculate a standard curve from the test results of the standard solutions. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Make colorful concentrated and dilute solutions and. Vernier Beer's Law Lab.
From www.youtube.com
Beer's Law YouTube Vernier Beer's Law Lab This experiment is #11 of investigating chemistry through inquiry. Determining the concentration of a species using a vernier spectrometer. The experiment in the book includes student instructions as well as. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! The concentration of an unknown niso4. Calculate a standard curve from. Vernier Beer's Law Lab.
From www.studocu.com
Beer's Law Experiment Experiment 4 Beer’s Law. Purpose The purpose of this lab is to Vernier Beer's Law Lab In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. Determine the order of reaction by measuring the absorbance of a reactant at a. The experiment in the book includes student instructions as well as. To use spectroscopy to prepare a beer’s law plot of. The concentration of an unknown niso4. Make colorful concentrated. Vernier Beer's Law Lab.
From studylib.net
Beer's Law Lab Vernier Beer's Law Lab Use the beer's law plot to determine the concentration of an unknown analyte. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The concentration of an unknown niso4. Determine the order of reaction by measuring the absorbance of a reactant at a. Make colorful concentrated and dilute solutions and explore how much light. Vernier Beer's Law Lab.
From studylib.net
Lab 7 Beer's Law Vernier Beer's Law Lab Determining the concentration of a species using a vernier spectrometer. Use the beer's law plot to determine the concentration of an unknown analyte. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! The concentration of an unknown niso4. In this experiment, you will prepare and test the absorbance of five. Vernier Beer's Law Lab.
From studylib.net
BeerLambert Law using Vernier/Ocean Optics Spectrometer (VSPEC) INTRODUCTION Vernier Beer's Law Lab In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate solutions. This experiment is #11 of investigating chemistry through inquiry. The direct relationship between absorbance and concentration for a solution is known as beer’s law. To use spectroscopy to prepare a beer’s law plot of. Make colorful concentrated and dilute solutions and explore how. Vernier Beer's Law Lab.
From www.youtube.com
Beer's Law Overview YouTube Vernier Beer's Law Lab The concentration of an unknown niso4. This experiment is #11 of investigating chemistry through inquiry. The direct relationship between absorbance and concentration for a solution is known as beer’s law. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! Determining the concentration of a species using a vernier spectrometer. The. Vernier Beer's Law Lab.
From laboratorysciences.blogspot.com
Beer's Law The Laboratory Vernier Beer's Law Lab Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! To use spectroscopy to prepare a beer’s law plot of. The experiment in the book includes student instructions as well as. The concentration of an unknown niso4. Use the beer's law plot to determine the concentration of an unknown analyte. Determining. Vernier Beer's Law Lab.
From www.verniercanada.ca
Vernier Spectral Analysis® Vernier Canada Vernier Beer's Law Lab In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The experiment in the book includes student instructions as well as. The concentration of an unknown niso4. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! The direct relationship between absorbance and concentration for. Vernier Beer's Law Lab.
From studylib.net
Beer's Law Lab Vernier Beer's Law Lab The direct relationship between absorbance and concentration for a solution is known as beer’s law. Use the beer's law plot to determine the concentration of an unknown analyte. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate. Vernier Beer's Law Lab.
From www.youtube.com
Beer's Law using a Vernier Go Direct Spectrovis Plus YouTube Vernier Beer's Law Lab Calculate a standard curve from the test results of the standard solutions. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! The concentration of an unknown niso4. The direct relationship between absorbance and concentration for a solution is known as beer’s law. In most experiments, molar absorptivity (ε) and the. Vernier Beer's Law Lab.
From www.studocu.com
Vernier calipers Experiments EXPERIMENT 1. Vernier CaliperS Aim To determine the volume of a Vernier Beer's Law Lab In this experiment, you will prepare and test the absorbance of five standard copper (ii) sulfate solutions. Calculate a standard curve from the test results of the standard solutions. Determine the order of reaction by measuring the absorbance of a reactant at a. The experiment in the book includes student instructions as well as. The concentration of an unknown niso4.. Vernier Beer's Law Lab.
From www.youtube.com
Spectrophotometry and Beer's Law YouTube Vernier Beer's Law Lab This experiment is #11 of investigating chemistry through inquiry. To use spectroscopy to prepare a beer’s law plot of. The direct relationship between absorbance and concentration for a solution is known as beer’s law. The concentration of an unknown niso4. Calculate a standard curve from the test results of the standard solutions. Determine the order of reaction by measuring the. Vernier Beer's Law Lab.
From www.thoughtco.com
Beer's Law Definition and Equation Vernier Beer's Law Lab Determine the order of reaction by measuring the absorbance of a reactant at a. The concentration of an unknown niso4. Determining the concentration of a species using a vernier spectrometer. In most experiments, molar absorptivity (ε) and the length (b) are constant, therefore, absorbance (a) is. The direct relationship between absorbance and concentration for a solution is known as beer’s. Vernier Beer's Law Lab.
From www.youtube.com
Beer’s Law Lab Analysis YouTube Vernier Beer's Law Lab The experiment in the book includes student instructions as well as. Calculate a standard curve from the test results of the standard solutions. The concentration of an unknown niso4. Determining the concentration of a species using a vernier spectrometer. Make colorful concentrated and dilute solutions and explore how much light they absorb and transmit using a virtual spectrophotometer! In most. Vernier Beer's Law Lab.