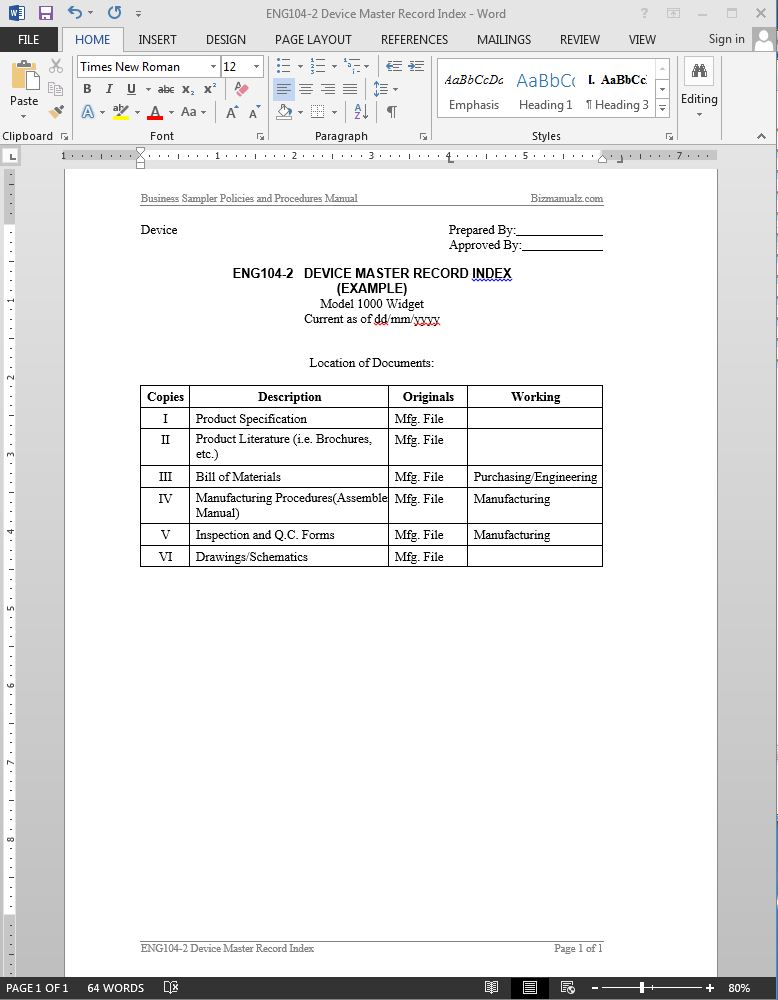
Device Master Record List . Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,.
from www.vrogue.co
device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. the device master record (dmr) contains appropriate drawings, material composition, specifications for the different.
Device Master Record Index Template vrogue.co
Device Master Record List when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Each manufacturer shall maintain device master records (dmr's). the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. § 820.181 device master record.
From matrixreq.com
What is the Device Master Record (DMR)? Device Master Record List device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). when your. Device Master Record List.
From www.presentationeze.com
Device Master Record DMR Information & Training.PresentationEZE Device Master Record List Each manufacturer shall maintain device master records (dmr's). device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. the device master record (dmr) contains appropriate drawings,. Device Master Record List.
From www.technia.co.uk
What is a Device Master Record? TECHNIA (UK) Device Master Record List master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. when. Device Master Record List.
From www.vrogue.co
Device Master Record Index Template vrogue.co Device Master Record List device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. Each. Device Master Record List.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID Device Master Record List device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. the device. Device Master Record List.
From www.presentationeze.com
Device Master Record (DMR) What needs to be recorded into the DMR Device Master Record List master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. device master. Device Master Record List.
From www.qualitymeddev.com
Device Master Record Overview of FDA Requiements Device Master Record List Each manufacturer shall maintain device master records (dmr's). the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. § 820.181 device master record. master files are created to help preserve the. Device Master Record List.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID Device Master Record List the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. Each manufacturer shall maintain device master records (dmr's). master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. device master record • maintain a device master record (dmr) • prepare and approve dmr. Device Master Record List.
From www.qualitymeddev.com
Device Master Record Overview of FDA Requiements Device Master Record List when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. device. Device Master Record List.
From www.technia.com
What is a Device Master Record? TECHNIA Device Master Record List § 820.181 device master record. the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. master files are created to help preserve the trade secrets of the ancillary medical device industry. Device Master Record List.
From www.technia.co.uk
What is a Device Master Record? TECHNIA (UK) Device Master Record List Each manufacturer shall maintain device master records (dmr's). the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. § 820.181 device master record. when your device is in production your dmr should. Device Master Record List.
From www.arenasolutions.com
Managing The Device Master Record (DMR) to Comply with 21 CFR Part 11 Device Master Record List master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). the device. Device Master Record List.
From www.slideserve.com
PPT Product Documentation PowerPoint Presentation, free download ID Device Master Record List master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. when your device is in production your dmr should be the definitive record of all your. Device Master Record List.
From studylib.net
Device Master Record Device Master Record List when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. . Device Master Record List.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID Device Master Record List when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. Each manufacturer shall maintain device master records (dmr's). the device master record (dmr) contains appropriate drawings,. Device Master Record List.
From www.scribd.com
Device Master Records PDF Specification (Technical Standard Device Master Record List master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. when. Device Master Record List.
From quality.eleapsoftware.com
Understanding the Importance of Device Master Record eLeaP Device Master Record List device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. § 820.181 device master record. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. the device master record (dmr) contains appropriate drawings, material composition,. Device Master Record List.
From www.johner-institut.de
Device Master Record DMR Auch für Software?!? Device Master Record List the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. when your device is in production your dmr. Device Master Record List.
From old.sermitsiaq.ag
Device Master Record Template Device Master Record List when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. master files are created to help preserve the trade secrets of the ancillary medical device. Device Master Record List.
From www.arenasolutions.com
Managing The Device Master Record (DMR) Arena Device Master Record List when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. § 820.181 device master record. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. device master record • maintain a device master record (dmr). Device Master Record List.
From fdacompliancehelp.blogspot.com
FDA Compliance Help October 2011 Device Master Record List § 820.181 device master record. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. device master record • maintain a device master record (dmr). Device Master Record List.
From www.technia.co.jp
What is a Device Master Record? TECHNIA (Japan) Device Master Record List the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. when. Device Master Record List.
From www.bizmanualz.com
Device Master Record Procedure Template Word Device Master Record List master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. § 820.181 device master record. when your device is in production your dmr should be. Device Master Record List.
From www.bizmanualz.com
Device Master Record Contents Template Word Device Master Record List § 820.181 device master record. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. when your device is in production your dmr should be. Device Master Record List.
From hardcoreqms.com
Device Master Records (DMR) for Medical Devices (2023) Device Master Record List the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. when. Device Master Record List.
From www.bizmanualz.com
Device Master Record Index Template Device Master Record List the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. Each manufacturer shall maintain device master records (dmr's). when your device is in production your dmr should be the definitive record of. Device Master Record List.
From www.instantgmp.com
Device Master Record Device Master Record List device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). the. Device Master Record List.
From www.aplyon.com
ISO 13485 Document Control Procedure Bundle Device Master Record List master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). when your device is in production your dmr should. Device Master Record List.
From denner-shop-test-web02.denner.ch
Device Master Record Template Device Master Record List device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. master files are created to help preserve the trade secrets of the ancillary medical device. Device Master Record List.
From www.bizmanualz.com
Device Master Record Index Template Word Device Master Record List § 820.181 device master record. when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. device master record • maintain a device master record (dmr). Device Master Record List.
From www.youtube.com
Preparing a Device Master Record (DMR) YouTube Device Master Record List when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. master files are created to help preserve the trade secrets of the ancillary medical device industry and at the same. the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. Each manufacturer. Device Master Record List.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID Device Master Record List the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. § 820.181 device master record. when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. device master record • maintain a device master record (dmr) • prepare and approve dmr in. Device Master Record List.
From www.inpaspages.com
Master list of Documents Device Master Record List Each manufacturer shall maintain device master records (dmr's). the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. master files are created to help preserve the trade secrets of the ancillary medical. Device Master Record List.
From www.instantgmp.com
Device Master Record Device Master Record List when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. device master record • maintain a device master record (dmr) • prepare and approve dmr in accordance with 21 cfr 820.40,. § 820.181 device master record. master files are created to help preserve the trade. Device Master Record List.
From www.arenasolutions.com
Managing The Device Master Record (DMR) Arena Device Master Record List when your device is in production your dmr should be the definitive record of all your manufacturing and product management processes. Each manufacturer shall maintain device master records (dmr's). the device master record (dmr) contains appropriate drawings, material composition, specifications for the different. master files are created to help preserve the trade secrets of the ancillary medical. Device Master Record List.