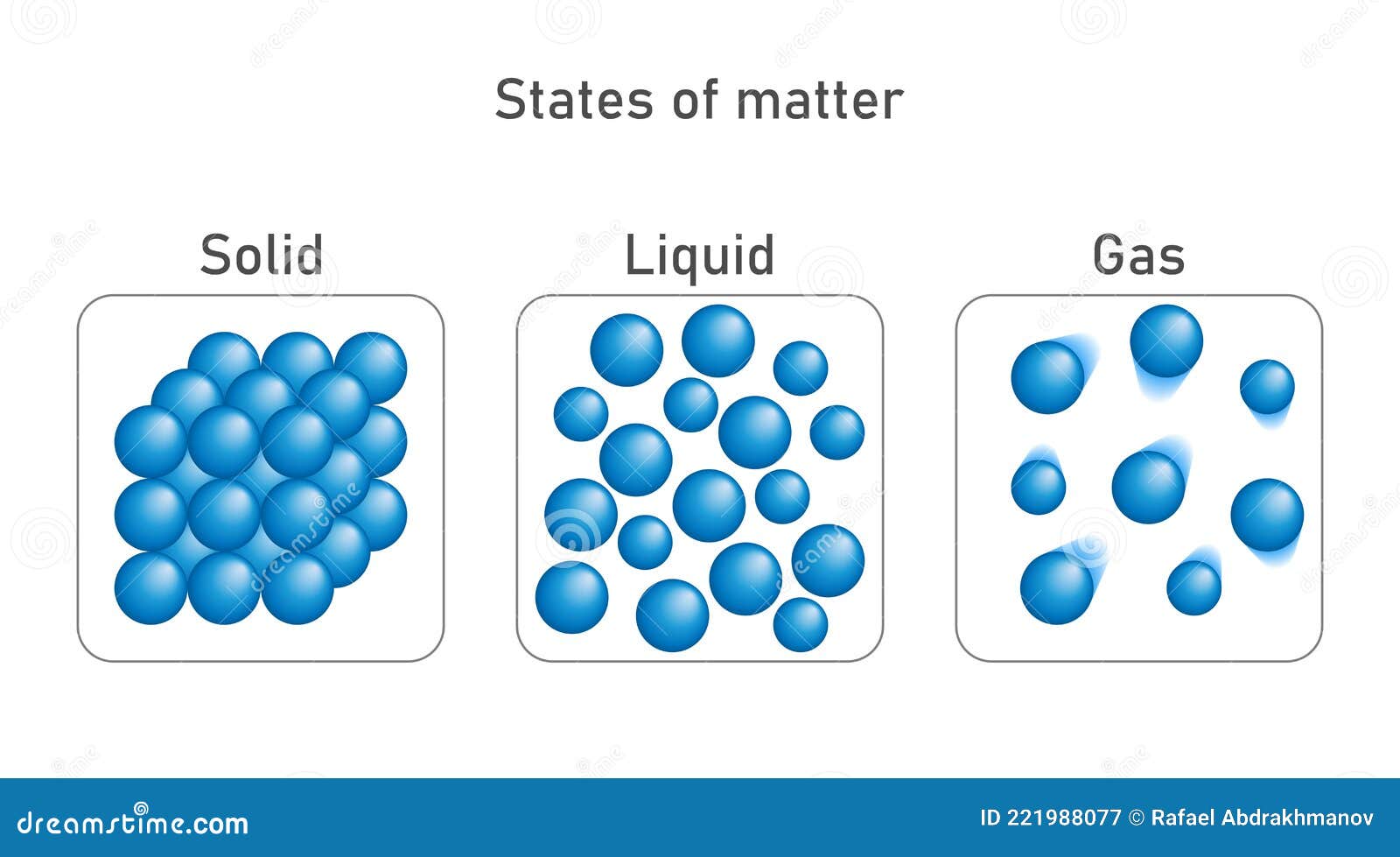
What Properties Of Solids Liquids And Gases Make Them Different And Similar . what is the difference between the movement of particles in liquids and the movement of particles in gases? Earlier in chapter 1, we briefly discussed the. in general covalent bonds determine: the table shows some of the properties of solids and why they are like this: The three states of matter can be represented by the particle model. Solids such as concrete are useful. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Solids, liquids and gases are all around us, they are the three main states. solids, liquids and gases. The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. All substances are made from.
from www.dreamstime.com
Earlier in chapter 1, we briefly discussed the. the table shows some of the properties of solids and why they are like this: The three states of matter can be represented by the particle model. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. Solids, liquids and gases are all around us, they are the three main states. Solids such as concrete are useful. The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. in general covalent bonds determine: in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. All substances are made from.
States of Matter. Vector Circles Infographic Illustration Stock Vector
What Properties Of Solids Liquids And Gases Make Them Different And Similar All substances are made from. what is the difference between the movement of particles in liquids and the movement of particles in gases? Earlier in chapter 1, we briefly discussed the. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. the table shows some of the properties of solids and why they are like this: The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. Solids such as concrete are useful. solids, liquids and gases. The three states of matter can be represented by the particle model. in general covalent bonds determine: All substances are made from. Solids, liquids and gases are all around us, they are the three main states.
From examplespedia.com
Difference between Solid Liquid and Gas ExamplesPedia What Properties Of Solids Liquids And Gases Make Them Different And Similar in general covalent bonds determine: in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. what is the difference between the movement of particles in liquids and the movement of particles in gases? Solids such as concrete are useful. Earlier in chapter 1, we briefly discussed the. The kinetic. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From mungfali.com
Solids Liquids Gases Chart What Properties Of Solids Liquids And Gases Make Them Different And Similar solids, liquids and gases. what is the difference between the movement of particles in liquids and the movement of particles in gases? All substances are made from. The three states of matter can be represented by the particle model. Earlier in chapter 1, we briefly discussed the. in general covalent bonds determine: a solid has definite. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.visionlearning.com
Properties of Liquids Chemistry Visionlearning What Properties Of Solids Liquids And Gases Make Them Different And Similar Solids, liquids and gases are all around us, they are the three main states. solids, liquids and gases. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. The three states of matter can be represented by the particle model. the table shows some of the properties of solids. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.primaryworks.co.uk
PowerPoint KS2 explanation on solids, liquids & gases KS2 primary What Properties Of Solids Liquids And Gases Make Them Different And Similar The three states of matter can be represented by the particle model. solids, liquids and gases. Solids, liquids and gases are all around us, they are the three main states. The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. the table shows some of the properties of solids and why they. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science What Properties Of Solids Liquids And Gases Make Them Different And Similar the table shows some of the properties of solids and why they are like this: All substances are made from. The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. Solids, liquids and gases. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From dxoodakes.blob.core.windows.net
Solids Liquids Gases Properties at Regina Tribble blog What Properties Of Solids Liquids And Gases Make Them Different And Similar what is the difference between the movement of particles in liquids and the movement of particles in gases? The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. the table shows some of the properties of solids and why they are like this: in general covalent bonds determine: Solids such as. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From dxowyevmd.blob.core.windows.net
What Are Some Properties Of Solids Liquids And Gases at Harry Hamilton blog What Properties Of Solids Liquids And Gases Make Them Different And Similar The three states of matter can be represented by the particle model. solids, liquids and gases. what is the difference between the movement of particles in liquids and the movement of particles in gases? Solids such as concrete are useful. the table shows some of the properties of solids and why they are like this: The kinetic. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From brainly.ph
using the 3 circle vendiagram and contrast the properties of What Properties Of Solids Liquids And Gases Make Them Different And Similar a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. Solids, liquids and gases are all around us, they are the three main. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From diagramlibkutshase6.z13.web.core.windows.net
Solid Liquid And Gas Particle Diagram What Properties Of Solids Liquids And Gases Make Them Different And Similar the table shows some of the properties of solids and why they are like this: The three states of matter can be represented by the particle model. what is the difference between the movement of particles in liquids and the movement of particles in gases? The kinetic molecular theory of gases gives a reasonably accurate description of the. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.breakingatom.com
States of Matter Solids, Liquids, and Gases What Properties Of Solids Liquids And Gases Make Them Different And Similar Solids such as concrete are useful. in general covalent bonds determine: The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. Solids, liquids and gases are all around us, they are the three main states. All substances are made from. Earlier in chapter 1, we briefly discussed the. the table shows some. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From sebschemistry.blogspot.com
IGCSE Edexcel Chemistry Help 1.1 understand the arrangement, movement What Properties Of Solids Liquids And Gases Make Them Different And Similar The three states of matter can be represented by the particle model. All substances are made from. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. solids, liquids and gases. the table shows some of the properties of solids and why they are like this: The kinetic molecular. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.slideshare.net
solids, liquids and gases What Properties Of Solids Liquids And Gases Make Them Different And Similar Solids, liquids and gases are all around us, they are the three main states. The three states of matter can be represented by the particle model. The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. solids, liquids and gases. in this tutorial, you will learn about the properties of the solid,. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From guidedbmudvoltairian.z13.web.core.windows.net
Venn Diagram For Solids Liquids And Gases What Properties Of Solids Liquids And Gases Make Them Different And Similar solids, liquids and gases. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. what is the difference between the movement of particles in liquids and the movement of particles in gases? All substances are made from. Earlier in chapter 1, we briefly discussed the. The three states of. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.youtube.com
States of Matter Solid, Liquid and Gas Chemistry YouTube What Properties Of Solids Liquids And Gases Make Them Different And Similar All substances are made from. Solids, liquids and gases are all around us, they are the three main states. in general covalent bonds determine: the table shows some of the properties of solids and why they are like this: a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From primaryleap.co.uk
Chemistry States Of Matter Level 1 activity for kids PrimaryLeap.co.uk What Properties Of Solids Liquids And Gases Make Them Different And Similar The three states of matter can be represented by the particle model. Solids such as concrete are useful. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. what is the difference between the movement of particles in liquids and the movement of particles in gases? All substances are made. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From itinerantmission.blogspot.com
Itinerant Mission 3 Physical States of Matter Solid Liquid Gas What Properties Of Solids Liquids And Gases Make Them Different And Similar in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. Solids, liquids and gases are all around us, they are the three main states. Solids such as concrete are useful. solids, liquids and gases. The three states of matter can be represented by the particle model. All substances are made. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From studylib.net
Chapter 11 Liquids and Solids A. Intermolecular Forces What Properties Of Solids Liquids And Gases Make Them Different And Similar Solids such as concrete are useful. Earlier in chapter 1, we briefly discussed the. in general covalent bonds determine: in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. Solids, liquids and gases are all around us, they are the three main states. The three states of matter can be. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.tutorix.com
Give three characteristics of solid liquid and gas Tutorix What Properties Of Solids Liquids And Gases Make Them Different And Similar The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. the table shows some of the properties of solids and why they are like this: Solids such as concrete are useful. All substances are made from. a solid has definite volume and shape, a liquid has a definite volume but no definite. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From allaboutchemistry123.blogspot.com
What are solid, liquid, and gases? All About Chemistry What Properties Of Solids Liquids And Gases Make Them Different And Similar solids, liquids and gases. Earlier in chapter 1, we briefly discussed the. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. All substances are made from. Solids such as concrete are useful. the table shows some of the properties. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.youtube.com
Difference Between Solid Liquid And Gases State Of Matter What Properties Of Solids Liquids And Gases Make Them Different And Similar the table shows some of the properties of solids and why they are like this: The three states of matter can be represented by the particle model. what is the difference between the movement of particles in liquids and the movement of particles in gases? in this tutorial, you will learn about the properties of the solid,. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.ase.org.uk
Solids, liquids and gases What Properties Of Solids Liquids And Gases Make Them Different And Similar a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. solids, liquids and gases. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. The kinetic molecular theory of gases gives a. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From socratic.org
What are examples of gases, liquids, and solids? Socratic What Properties Of Solids Liquids And Gases Make Them Different And Similar the table shows some of the properties of solids and why they are like this: All substances are made from. Earlier in chapter 1, we briefly discussed the. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. solids, liquids and gases. The three states of matter can be. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.teachoo.com
Properties of Solids, Liquids, Gases Compared Teachoo Science What Properties Of Solids Liquids And Gases Make Them Different And Similar Solids such as concrete are useful. the table shows some of the properties of solids and why they are like this: in general covalent bonds determine: what is the difference between the movement of particles in liquids and the movement of particles in gases? Earlier in chapter 1, we briefly discussed the. Solids, liquids and gases are. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.slideserve.com
PPT SOLIDS LIQUIDS GASES PowerPoint Presentation, free download ID What Properties Of Solids Liquids And Gases Make Them Different And Similar in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. solids, liquids and gases. what is the difference between the movement of particles in liquids and the movement of particles in gases? Solids, liquids and gases are all around us, they are the three main states. Earlier in chapter. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.youtube.com
States of Matter Solid Liquid Gas States of Matter drawing Different What Properties Of Solids Liquids And Gases Make Them Different And Similar Solids such as concrete are useful. what is the difference between the movement of particles in liquids and the movement of particles in gases? The three states of matter can be represented by the particle model. All substances are made from. the table shows some of the properties of solids and why they are like this: The kinetic. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.jove.com
11340.jpg What Properties Of Solids Liquids And Gases Make Them Different And Similar in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.teachoo.com
Difference between Solid, Liquid, Gas in Table Form Teachoo What Properties Of Solids Liquids And Gases Make Them Different And Similar in general covalent bonds determine: The three states of matter can be represented by the particle model. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. in this tutorial, you will learn about the properties of the solid, liquid,. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.pinterest.ph
Properties of Solids, Liquids, Gases Compared Teachoo Science What Properties Of Solids Liquids And Gases Make Them Different And Similar The three states of matter can be represented by the particle model. Earlier in chapter 1, we briefly discussed the. Solids such as concrete are useful. The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. Solids, liquids and gases are all around us, they are the three main states. in this tutorial,. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From andres-yersblogsparks.blogspot.com
Solids Liquids and Gases What Properties Of Solids Liquids And Gases Make Them Different And Similar in general covalent bonds determine: Earlier in chapter 1, we briefly discussed the. solids, liquids and gases. All substances are made from. Solids such as concrete are useful. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. the table shows some of the properties of solids and. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.expii.com
Arrangement of Particles in Phases of Matter — Comparison Expii What Properties Of Solids Liquids And Gases Make Them Different And Similar Solids, liquids and gases are all around us, they are the three main states. Earlier in chapter 1, we briefly discussed the. All substances are made from. in general covalent bonds determine: the table shows some of the properties of solids and why they are like this: The kinetic molecular theory of gases gives a reasonably accurate description. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.snexplores.org
Explainer What are the different states of matter? What Properties Of Solids Liquids And Gases Make Them Different And Similar Earlier in chapter 1, we briefly discussed the. what is the difference between the movement of particles in liquids and the movement of particles in gases? The kinetic molecular theory of gases gives a reasonably accurate description of the behavior of gases. a solid has definite volume and shape, a liquid has a definite volume but no definite. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.dreamstime.com
States of Matter. Vector Circles Infographic Illustration Stock Vector What Properties Of Solids Liquids And Gases Make Them Different And Similar in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. solids, liquids and gases. the table shows some of the properties of solids and why they are like this: a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From stock.adobe.com
states of matter solids liquids and gases. Matter appears in three What Properties Of Solids Liquids And Gases Make Them Different And Similar The three states of matter can be represented by the particle model. solids, liquids and gases. a solid has definite volume and shape, a liquid has a definite volume but no definite shape, and a gas has neither a definite volume nor shape. the table shows some of the properties of solids and why they are like. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From sciencenotes.org
10 Examples of Solids, Liquids, Gases, and Plasma What Properties Of Solids Liquids And Gases Make Them Different And Similar what is the difference between the movement of particles in liquids and the movement of particles in gases? the table shows some of the properties of solids and why they are like this: The three states of matter can be represented by the particle model. in general covalent bonds determine: Solids, liquids and gases are all around. What Properties Of Solids Liquids And Gases Make Them Different And Similar.
From www.slideshare.net
Unit 1 Notes What Properties Of Solids Liquids And Gases Make Them Different And Similar Solids such as concrete are useful. All substances are made from. The three states of matter can be represented by the particle model. Solids, liquids and gases are all around us, they are the three main states. in this tutorial, you will learn about the properties of the solid, liquid, and gas phases of matter. what is the. What Properties Of Solids Liquids And Gases Make Them Different And Similar.