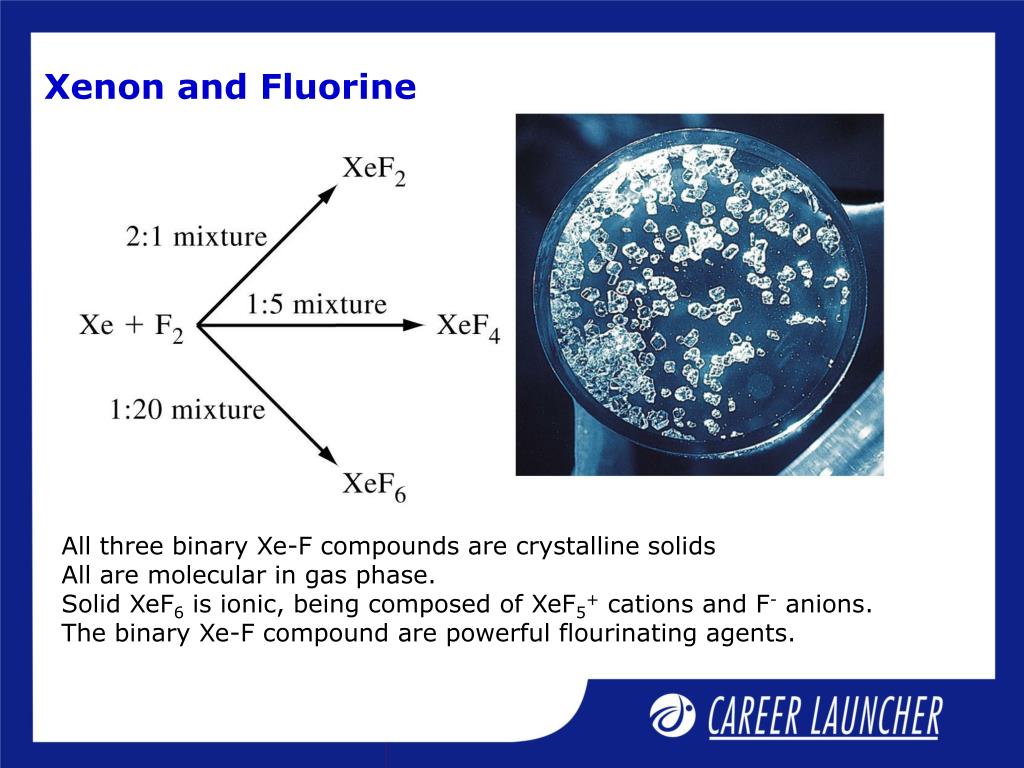
Why Does Xenon React With Fluorine . Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Its outer electrons then become a target for other. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Xenon can be brought to react with fluorine, f 2 [4]: Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Xe (g) + f 2 (g) xef 2 (s) xe.
from www.slideserve.com
Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Xe (g) + f 2 (g) xef 2 (s) xe. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Its outer electrons then become a target for other. Xenon can be brought to react with fluorine, f 2 [4]:
PPT Chemistry PowerPoint Presentation, free download ID650195
Why Does Xenon React With Fluorine Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Xe (g) + f 2 (g) xef 2 (s) xe. Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Its outer electrons then become a target for other. Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Xenon can be brought to react with fluorine, f 2 [4]: This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus.
From cen.watchglass.org
The Watch Glass “Inert” Xenon Reacts with Fluorine Top, Xenon... Why Does Xenon React With Fluorine This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Its outer electrons then become a target for other. Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Xenon reacts with fluorine due to its larger atomic size. Why Does Xenon React With Fluorine.
From www.doubtnut.com
Give reasons which prompted Bartlett to react xenon with fluorine to f Why Does Xenon React With Fluorine Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xenon can be brought to react with fluorine, f 2 [4]: Its outer electrons then become a target for. Why Does Xenon React With Fluorine.
From www.numerade.com
SOLVED The illustration to the left represents a mixture of xenon Why Does Xenon React With Fluorine Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xenon can be brought to react with fluorine, f 2 [4]: Noble gases form. Why Does Xenon React With Fluorine.
From www.numerade.com
SOLVED (a) Why does xenon react with fluorine, whereas neon does not Why Does Xenon React With Fluorine This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xenon can be brought to react with fluorine, f 2 [4]: Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Noble gases form compounds with fluorine and oxygen only because fluorine and. Why Does Xenon React With Fluorine.
From www.numerade.com
SOLVED Empirical Formula Prelab In 1970, xenon was made to react with Why Does Xenon React With Fluorine Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Xe (g) + f 2 (g) xef 2 (s) xe. Xenon can be brought to react with fluorine, f 2 [4]: This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the. Why Does Xenon React With Fluorine.
From www.slideserve.com
PPT CHEMISTRY 1000 PowerPoint Presentation, free download ID6083445 Why Does Xenon React With Fluorine Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Xenon can be brought to react with fluorine, f 2 [4]: This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xenon forms compounds because its inner electrons screen. Why Does Xenon React With Fluorine.
From www.numerade.com
SOLVED Xenon gas reacts with fluorine gas to produce xenon Why Does Xenon React With Fluorine Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Xe (g) + f 2 (g) xef 2 (s) xe. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xenon can be brought to react with fluorine, f. Why Does Xenon React With Fluorine.
From www.numerade.com
SOLVEDWhy does xenon react with fluorine, whereas neon does not? Why Does Xenon React With Fluorine Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Xe (g) + f 2 (g) xef 2 (s) xe. This paper presents a brief review of the basic information regarding xenon(ii) fluoride. Why Does Xenon React With Fluorine.
From www.youtube.com
Compounds of Xenon with oxygen and fluorine YouTube Why Does Xenon React With Fluorine Xenon can be brought to react with fluorine, f 2 [4]: Its outer electrons then become a target for other. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis. Why Does Xenon React With Fluorine.
From www.youtube.com
Xenon fluorine compounds YouTube Why Does Xenon React With Fluorine Xe (g) + f 2 (g) xef 2 (s) xe. Xenon can be brought to react with fluorine, f 2 [4]: Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most. Why Does Xenon React With Fluorine.
From www.numerade.com
⏩SOLVED(a) Why does xenon react with fluorine, whereas neon does Why Does Xenon React With Fluorine Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a. Why Does Xenon React With Fluorine.
From www.numerade.com
The illustration to the left represents a mixture of xenon brown and Why Does Xenon React With Fluorine Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a. Why Does Xenon React With Fluorine.
From www.bartleby.com
Answered The illustration to the left represents… bartleby Why Does Xenon React With Fluorine Xe (g) + f 2 (g) xef 2 (s) xe. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Its outer electrons then become a target for other.. Why Does Xenon React With Fluorine.
From www.numerade.com
SOLVEDDirect reaction of fluorine with xenon produces three different Why Does Xenon React With Fluorine Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Its outer electrons then become a target for other. Xenon can be brought to react with fluorine, f 2 [4]: Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide. Why Does Xenon React With Fluorine.
From byjus.com
A 2g sample of xenon reacts with fluorine. The mass of compound Why Does Xenon React With Fluorine Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Xe (g) + f 2 (g) xef 2 (s) xe. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such. Why Does Xenon React With Fluorine.
From www.britannica.com
Xenon Definition, Properties, Atomic Mass, Compounds, & Facts Why Does Xenon React With Fluorine Its outer electrons then become a target for other. Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xe (g) + f 2 (g) xef 2 (s) xe.. Why Does Xenon React With Fluorine.
From www.numerade.com
The illustration to the left represents a mixture of xenon (brown ) and Why Does Xenon React With Fluorine Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Its outer electrons then become a target for other. Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as. Why Does Xenon React With Fluorine.
From www.numerade.com
SOLVED Although xenon is a “noble gas,” it reacts with fluorine and Why Does Xenon React With Fluorine Its outer electrons then become a target for other. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Xe (g) + f 2 (g) xef 2 (s) xe. Xenon can react with. Why Does Xenon React With Fluorine.
From www.slideserve.com
PPT CHEMISTRY 1000 PowerPoint Presentation, free download ID6083445 Why Does Xenon React With Fluorine Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Xe (g) + f 2. Why Does Xenon React With Fluorine.
From www.numerade.com
SOLVED(a) Why does xenon react with fluorine, whereas neon does not Why Does Xenon React With Fluorine Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Xe (g) + f 2 (g) xef 2 (s) xe. Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen. Why Does Xenon React With Fluorine.
From www.numerade.com
SOLVED Xenon gas and fluorine gas react over a platinum catalyst to Why Does Xenon React With Fluorine Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Its outer electrons then become a target for other. Xe (g) + f 2 (g) xef 2 (s) xe. Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Xenon reacts with fluorine due to its larger atomic. Why Does Xenon React With Fluorine.
From www.numerade.com
SOLVED (a) Why does xenon react with fluorine, whereas neon does not Why Does Xenon React With Fluorine Its outer electrons then become a target for other. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Xe (g) + f 2 (g) xef 2 (s) xe.. Why Does Xenon React With Fluorine.
From www.chegg.com
Solved Xenon can react with oxygen and fluorine to form Why Does Xenon React With Fluorine This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Its outer electrons then become a target for other. Noble gases form compounds with fluorine and oxygen only because. Why Does Xenon React With Fluorine.
From www.slideserve.com
PPT The Noble Gases PowerPoint Presentation, free download ID6532925 Why Does Xenon React With Fluorine Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Xenon can be brought to react with fluorine, f 2 [4]: Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). This paper presents a brief review of the basic information regarding xenon(ii) fluoride. Why Does Xenon React With Fluorine.
From www.numerade.com
Xenon and fluorine will react to form binary compounds when a mixture Why Does Xenon React With Fluorine Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Xe (g) + f 2 (g) xef 2 (s) xe. Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Xenon can be brought to react with fluorine, f 2 [4]: This paper presents. Why Does Xenon React With Fluorine.
From www.slideserve.com
PPT Group 18 Elements Noble Gases PowerPoint Presentation, free Why Does Xenon React With Fluorine Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Xe (g) + f 2 (g) xef 2 (s) xe. Xenon can be brought to react with fluorine, f. Why Does Xenon React With Fluorine.
From www.toppr.com
Xenon form compounds with fluorine under different conditions. The Why Does Xenon React With Fluorine Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. This. Why Does Xenon React With Fluorine.
From www.examples.com
Xenon (Xe) Definition, Preparation, Properties, Uses, Compounds Why Does Xenon React With Fluorine Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Xe (g) + f 2 (g) xef 2 (s) xe. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence. Why Does Xenon React With Fluorine.
From www.numerade.com
SOLVEDThe reaction of three molecules of fluorine gas with a Xe atom Why Does Xenon React With Fluorine Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Its outer electrons then become a target for other. Xenon can be brought to react with fluorine, f 2 [4]: Xenon reacts with fluorine due to its larger. Why Does Xenon React With Fluorine.
From www.doubtnut.com
[Malayalam] Xenon react with fluorine to form 3 types of fluorides. Wh Why Does Xenon React With Fluorine Noble gases form compounds with fluorine and oxygen only because fluorine and oxygen are the most electronegative elements. Its outer electrons then become a target for other. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. This paper presents a brief review of the basic information. Why Does Xenon React With Fluorine.
From www.slideserve.com
PPT Chemistry PowerPoint Presentation, free download ID650195 Why Does Xenon React With Fluorine This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Its outer electrons then become a target for other. Xe (g) + f 2 (g) xef 2 (s) xe. Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4).. Why Does Xenon React With Fluorine.
From www.slideserve.com
PPT Group 18 Elements Noble Gases PowerPoint Presentation ID2168487 Why Does Xenon React With Fluorine Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical.. Why Does Xenon React With Fluorine.
From www.numerade.com
The illustration to the left represents a mixture of xenon (brown ) and Why Does Xenon React With Fluorine Its outer electrons then become a target for other. Xe (g) + f 2 (g) xef 2 (s) xe. Xenon can be brought to react with fluorine, f 2 [4]: Xenon reacts with fluorine due to its larger atomic size and the greater shielding effect on its valence electrons, which allows the highly. Xenon forms compounds because its inner electrons. Why Does Xenon React With Fluorine.
From www.chegg.com
Solved 3. Xenon can be made to react with fluorine gas at Why Does Xenon React With Fluorine Xenon can react with fluorine to form xenon hexafluoride (xef6) and with oxygen to form xenon tetroxide (xeo4). Xe (g) + f 2 (g) xef 2 (s) xe. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xenon forms compounds because its inner electrons screen the. Why Does Xenon React With Fluorine.
From www.doubtnut.com
[Odia] Why does xenon form compounds only with fluorine and oxygen Why Does Xenon React With Fluorine Xenon forms compounds because its inner electrons screen the outer electrons from the nucleus. Its outer electrons then become a target for other. This paper presents a brief review of the basic information regarding xenon(ii) fluoride such as the synthesis of a pure compound, its physical. Xe (g) + f 2 (g) xef 2 (s) xe. Xenon can react with. Why Does Xenon React With Fluorine.