Carbon Tetrafluoride Flame Test . The principle of the test is. the objectives of this lab are to: 50 rows a flame test is relatively quick test for the presence of some elements in a sample. The technique is archaic and of questionable. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. the flame test is an easy experiment to set up and is often conducted in science classes. the characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for.
from peda.net
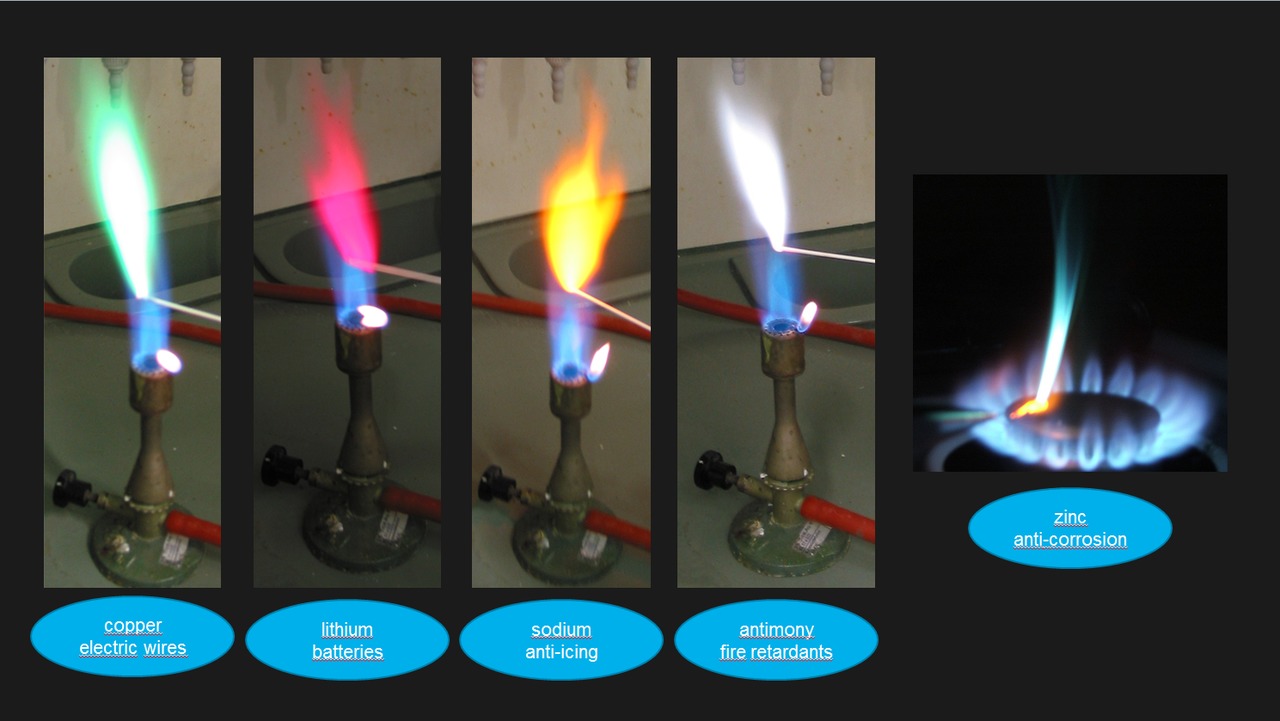
the objectives of this lab are to: The principle of the test is. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. 50 rows a flame test is relatively quick test for the presence of some elements in a sample. the flame test is an easy experiment to set up and is often conducted in science classes. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. The technique is archaic and of questionable.
Flame tests
Carbon Tetrafluoride Flame Test 50 rows a flame test is relatively quick test for the presence of some elements in a sample. The principle of the test is. The technique is archaic and of questionable. the objectives of this lab are to: this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. the flame test is an easy experiment to set up and is often conducted in science classes. 50 rows a flame test is relatively quick test for the presence of some elements in a sample. the characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound.
From worksheetmediascalar.z22.web.core.windows.net
Procedure Of Flame Test Carbon Tetrafluoride Flame Test Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. the characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for. 50 rows a flame test is relatively quick test for the presence of some elements. Carbon Tetrafluoride Flame Test.
From www.chegg.com
Solved Consider a sample of carbon tetrafluoride that Carbon Tetrafluoride Flame Test Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the objectives of this lab are to: the characteristic colors of light produced when substances. Carbon Tetrafluoride Flame Test.
From lessonlibspelunking.z22.web.core.windows.net
Procedure Of Flame Test Carbon Tetrafluoride Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. the characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for. this page describes how to perform a flame test. Carbon Tetrafluoride Flame Test.
From www.chegg.com
Solved 41. Carbon tetrafluoride, CF4, and sulfur Carbon Tetrafluoride Flame Test the flame test is an easy experiment to set up and is often conducted in science classes. 50 rows a flame test is relatively quick test for the presence of some elements in a sample. The principle of the test is. this page describes how to perform a flame test for a range of metal ions, and. Carbon Tetrafluoride Flame Test.
From maghsci.blogspot.com
Magh Sci LC Chemistry Flame Tests Carbon Tetrafluoride Flame Test Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. 50 rows a flame test is relatively quick test for the presence of some elements in a sample. the flame test is. Carbon Tetrafluoride Flame Test.
From www.yanxachem.com
China Carbon Tetrafluoride factory and manufacturers YANXA Carbon Tetrafluoride Flame Test Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. the objectives of this lab are to: this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the characteristic colors of light produced when substances are heated. Carbon Tetrafluoride Flame Test.
From ar.inspiredpencil.com
Carbon Tetrafluoride Lewis Structure Carbon Tetrafluoride Flame Test 50 rows a flame test is relatively quick test for the presence of some elements in a sample. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. The principle of the test is. to perform flame tests of metal cations in order to observe their characteristic colors, to match the. Carbon Tetrafluoride Flame Test.
From www.chemistrylearner.com
Polarity of Carbon Tetrafluoride (CF4) Carbon Tetrafluoride Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. 50 rows a flame test is relatively quick test for the presence of some elements in a. Carbon Tetrafluoride Flame Test.
From shcmc123.en.made-in-china.com
Cylinder Gas Fire Extinguishing Electrical Insulation Usage Tracer Gas Carbon Tetrafluoride Flame Test the characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for. The technique is archaic and of questionable. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. the flame. Carbon Tetrafluoride Flame Test.
From sciencelessonsthatrock.com
Chemistry Flame Test Lab Science Lessons That Rock Carbon Tetrafluoride Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. this page describes how to perform a flame test for a range of metal ions,. Carbon Tetrafluoride Flame Test.
From www.chegg.com
Solved Table 21. Flame test of solid compounds. Sample LiCI Carbon Tetrafluoride Flame Test 50 rows a flame test is relatively quick test for the presence of some elements in a sample. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. the flame test is an easy experiment to set up and is often conducted in. Carbon Tetrafluoride Flame Test.
From www.youtube.com
CF4 (Carbon tetrafluoride) Molecular Geometry, Bond Angles & Electron Carbon Tetrafluoride Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. The technique is archaic and of questionable. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Flame tests are used. Carbon Tetrafluoride Flame Test.
From ar.inspiredpencil.com
Carbon Tetrafluoride Lewis Structure Carbon Tetrafluoride Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. The technique is archaic and of questionable. the objectives of this lab are to: to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed. Carbon Tetrafluoride Flame Test.
From www.shutterstock.com
Chemical Structure Carbon Tetrafluoride Tetrafluoromethane Cf4 Stock Carbon Tetrafluoride Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. 50 rows a flame test is relatively quick test for the presence of some elements in a sample. the characteristic colors of light produced when substances are heated in the flame of a. Carbon Tetrafluoride Flame Test.
From lambdageeks.com
5 Carbon Tetrafluoride Uses Facts You Should Know! LAMBDAGEEKS Carbon Tetrafluoride Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. the objectives of this lab are to: The principle of the test is. this. Carbon Tetrafluoride Flame Test.
From www.xenon-gas.com
Carbon Tetrafluoride, CF4 Specialty Gas Chengdu Xenon Tritium Carbon Tetrafluoride Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. the objectives of this lab are to: Perform flame tests of metal cations in order. Carbon Tetrafluoride Flame Test.
From peda.net
Flame tests Carbon Tetrafluoride Flame Test Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. The technique is archaic and of questionable. the characteristic colors of light produced when substances are heated in the flame of a gas. Carbon Tetrafluoride Flame Test.
From scienceready.com.au
Atomic Emission Spectroscopy & Flame Test HSC Chemistry Science Ready Carbon Tetrafluoride Flame Test the objectives of this lab are to: the flame test is an easy experiment to set up and is often conducted in science classes. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. the characteristic colors of light produced when substances are heated in the flame of. Carbon Tetrafluoride Flame Test.
From www.alamyimages.fr
Test de flamme. Procédé en chimie analytique pour détecter la présence Carbon Tetrafluoride Flame Test to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. the objectives of this lab are to: the characteristic colors of light produced when substances are. Carbon Tetrafluoride Flame Test.
From www.chegg.com
Solved Consider a sample of carbon tetrafluoride that Carbon Tetrafluoride Flame Test Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. The technique is archaic and of questionable. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the characteristic colors of light produced when substances are. Carbon Tetrafluoride Flame Test.
From www.youtube.com
[Special gas]——Carbon Tetrafluoride (CF4) product introduction video Carbon Tetrafluoride Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the flame test is an easy experiment to set up and is often conducted in science classes. The principle of the test is. the objectives of this lab are to: Flame tests are used. Carbon Tetrafluoride Flame Test.
From www.youtube.com
CF4 Lewis Structure (Carbon Tetrafluoride) YouTube Carbon Tetrafluoride Flame Test The principle of the test is. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. 50 rows a flame test is relatively quick test for the presence. Carbon Tetrafluoride Flame Test.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Carbon Tetrafluoride Flame Test The technique is archaic and of questionable. the flame test is an easy experiment to set up and is often conducted in science classes. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. Flame tests are used to identify the presence of a relatively. Carbon Tetrafluoride Flame Test.
From www.youtube.com
Carbon tetrafluorideHow to write chemical formula of carbon Carbon Tetrafluoride Flame Test Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. 50 rows a flame test is relatively quick test for the presence of some elements in a sample. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises.. Carbon Tetrafluoride Flame Test.
From www.numerade.com
SOLVED Consider a sample of carbon tetrafluoride that contains 1.47 Carbon Tetrafluoride Flame Test Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. The principle of the test is. Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. to perform flame tests of metal cations in order to observe their characteristic colors, to match the. Carbon Tetrafluoride Flame Test.
From learningzoneamorytyvm.z14.web.core.windows.net
Procedure Of Flame Test Carbon Tetrafluoride Flame Test 50 rows a flame test is relatively quick test for the presence of some elements in a sample. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. to perform flame tests of metal cations in order to observe their characteristic colors, to match. Carbon Tetrafluoride Flame Test.
From sciencenotes.org
Flame Test Colors and Procedure (Chemistry) Carbon Tetrafluoride Flame Test the characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for. the objectives of this lab are to: the flame test is an easy experiment to set up and is often conducted in science classes. The principle of the test is. Flame tests are. Carbon Tetrafluoride Flame Test.
From www.chegg.com
Solved Consider a sample of carbon tetrafluoride that Carbon Tetrafluoride Flame Test The principle of the test is. 50 rows a flame test is relatively quick test for the presence of some elements in a sample. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. the objectives of this lab are to: this. Carbon Tetrafluoride Flame Test.
From www.dreamstime.com
Tetrafluoromethane or Carbon Tetrafluoride, CF4 Greenhouse Gas Molecule Carbon Tetrafluoride Flame Test Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. the objectives of this lab are to: this page describes how to perform a. Carbon Tetrafluoride Flame Test.
From www.thoughtco.com
How to Do a Flame Test for Qualitative Analysis Carbon Tetrafluoride Flame Test The principle of the test is. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. 50 rows a flame test is relatively quick test for the presence of some elements in a sample. Perform flame tests of metal cations in order to observe their. Carbon Tetrafluoride Flame Test.
From www.youtube.com
Flame Tests Chemistry Practicals YouTube Carbon Tetrafluoride Flame Test 50 rows a flame test is relatively quick test for the presence of some elements in a sample. the flame test is an easy experiment to set up and is often conducted in science classes. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color. Carbon Tetrafluoride Flame Test.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记2.2.5 Flame Tests翰林国际教育 Carbon Tetrafluoride Flame Test this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for. Flame tests are used to identify the presence of a relatively. Carbon Tetrafluoride Flame Test.
From cekrwhnq.blob.core.windows.net
Carbon Tetrachloride Flame Test at Sebastian Aguila blog Carbon Tetrafluoride Flame Test Flame tests are used to identify the presence of a relatively small number of metal ions in a compound. the objectives of this lab are to: to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. this page describes how to perform a. Carbon Tetrafluoride Flame Test.
From knowledge.carolina.com
Flame Test Colors and BrightLine Emission Spectra for Metal Chlorides Carbon Tetrafluoride Flame Test Perform flame tests of metal cations in order to observe their characteristic colors, perform calculations to determine. to perform flame tests of metal cations in order to observe their characteristic colors, to match the flame colors observed to an appropriate wavelength. this page describes how to perform a flame test for a range of metal ions, and briefly. Carbon Tetrafluoride Flame Test.
From en.wikipedia.org
Carbon tetrafluoride Wikipedia Carbon Tetrafluoride Flame Test the characteristic colors of light produced when substances are heated in the flame of a gas burner are the basis of flame tests for. this page describes how to perform a flame test for a range of metal ions, and briefly discusses how the flame color arises. the flame test is an easy experiment to set up. Carbon Tetrafluoride Flame Test.