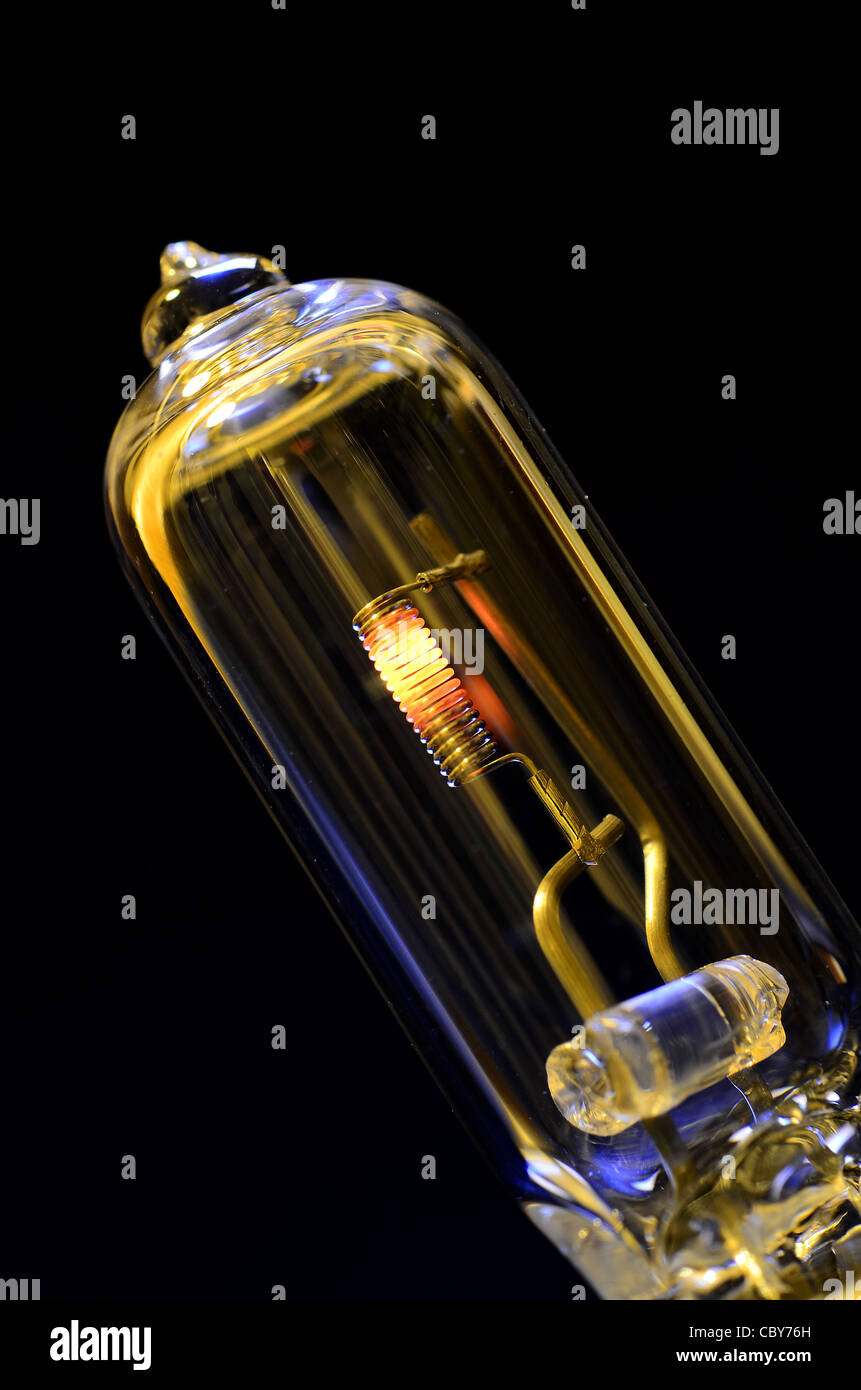
Tungsten Light Bulb Gas . How a tungsten halogen lamp works: the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint. the most common noble gases used in light bulbs are xenon, krypton, and argon. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation.
from www.alamy.com
as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. the most common noble gases used in light bulbs are xenon, krypton, and argon. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. How a tungsten halogen lamp works: 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint.
Tungsten Light Bulb Stock Photo Alamy
Tungsten Light Bulb Gas the most common noble gases used in light bulbs are xenon, krypton, and argon. the most common noble gases used in light bulbs are xenon, krypton, and argon. How a tungsten halogen lamp works: Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint. the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation.
From www.dreamstime.com
Traditional Tungsten Light Bulb Isolated on White Stock Photo Image Tungsten Light Bulb Gas 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. a halogen lamp also uses a tungsten filament,. Tungsten Light Bulb Gas.
From www.alamy.com
Glowing traditional Tungsten light bulb in physics class Stock Photo Tungsten Light Bulb Gas the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. Modern halogen. Tungsten Light Bulb Gas.
From 1000bulbs.com
25W Antique Edison Light Bulb 3 Loop Tungsten Filament Tungsten Light Bulb Gas a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. as the filament burns, tungsten particles. Tungsten Light Bulb Gas.
From www.dreamstime.com
Traditional Tungsten Light Bulb Isolated on White Stock Photo Image Tungsten Light Bulb Gas a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. How a tungsten halogen lamp works: the most common noble gases used in light bulbs are xenon, krypton, and argon. Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint. as the. Tungsten Light Bulb Gas.
From edisonlightglobes.com
Incandescent tungsten E27 25W clear round bulb Tungsten Light Bulb Gas the most common noble gases used in light bulbs are xenon, krypton, and argon. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. the only way you can get a tungsten bulb to. Tungsten Light Bulb Gas.
From www.photoflex.com
Starlite Tungsten Lamp / Light Bulb Photoflex Tungsten Light Bulb Gas the most common noble gases used in light bulbs are xenon, krypton, and argon. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. a halogen lamp. Tungsten Light Bulb Gas.
From www.alamy.com
An isolated tungsten light bulb turned on Stock Photo Alamy Tungsten Light Bulb Gas 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. Modern. Tungsten Light Bulb Gas.
From parcoscientific.com
Tungsten Light Bulb 30W, 110V Light Bulbs Parts & Accessories Tungsten Light Bulb Gas a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint. How a tungsten halogen lamp works: a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope.. Tungsten Light Bulb Gas.
From www.ebay.com
E27 40W Vintage Retro Filament Edison Tungsten Light Bulb Antique Lamp Tungsten Light Bulb Gas the most common noble gases used in light bulbs are xenon, krypton, and argon. How a tungsten halogen lamp works: a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. Modern halogen. Tungsten Light Bulb Gas.
From electricalmag.com
What is Tungsten Light Bulb? ElectricalMag Tungsten Light Bulb Gas the most common noble gases used in light bulbs are xenon, krypton, and argon. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. 1) tungsten atoms (yellow) vaporize from the filament and would. Tungsten Light Bulb Gas.
From www.dreamstime.com
Trun On Tungsten Light Bulb, Realistic Photo Image Stock Image Image Tungsten Light Bulb Gas a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. How a tungsten halogen lamp works:. Tungsten Light Bulb Gas.
From www.alamy.com
Traditional tungsten light bulb isolated on a white studio background Tungsten Light Bulb Gas How a tungsten halogen lamp works: 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. the most common noble gases used in light bulbs are xenon, krypton, and argon. as the filament burns,. Tungsten Light Bulb Gas.
From www.sciencephoto.com
Tungsten filament candle light bulbs Stock Image C036/1164 Tungsten Light Bulb Gas Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. the only way you can. Tungsten Light Bulb Gas.
From www.sylprotec.com
Tungsten Light bulb 24 V 18 W for emergency light unit. Tungsten Light Bulb Gas as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. How a tungsten halogen lamp works: the most. Tungsten Light Bulb Gas.
From www.bulbamerica.com
OSRAM 2000w 115v BVW 3200k T9.5 Tungsten Single Ended Halogen Light Bu Tungsten Light Bulb Gas 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. the most common noble gases used in light bulbs are xenon, krypton, and argon. a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. as the filament burns, tungsten particles separate from the. Tungsten Light Bulb Gas.
From pinoyeshop.com
E27 110130v Edison Tungsten Filament Vintage Light Bulb G80 Threaded Tungsten Light Bulb Gas the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. the. Tungsten Light Bulb Gas.
From www.dreamstime.com
Set of Traditional Tungsten Light Bulbs Isolated on White Stock Image Tungsten Light Bulb Gas the most common noble gases used in light bulbs are xenon, krypton, and argon. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. a mix of halogen gas, nitrogen, and argon. Tungsten Light Bulb Gas.
From www.artnews.com
Best Tungsten Light Bulbs Tungsten Light Bulb Gas a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. How. Tungsten Light Bulb Gas.
From www.alamy.com
Traditional tungsten light bulb isolated on a white studio background Tungsten Light Bulb Gas the most common noble gases used in light bulbs are xenon, krypton, and argon. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. a mix of halogen gas, nitrogen, and argon maintains high pressure. Tungsten Light Bulb Gas.
From www.ukdj.co.uk
Linear Tungsten Halogen Light Bulb 500w K5 254mm Tungsten Light Bulb Gas a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint. the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of.. Tungsten Light Bulb Gas.
From collections.museumsvictoria.com.au
Electric Lamp Tungsten Filament, G.E.C., 'Osram GasFilled', England Tungsten Light Bulb Gas a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. Modern halogen lamps often use bromine, which is. Tungsten Light Bulb Gas.
From www.alamy.com
lighted tungsten light bulb Stock Photo Alamy Tungsten Light Bulb Gas a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. How a tungsten halogen lamp works: the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. as the filament burns, tungsten particles separate from the filament, eventually. Tungsten Light Bulb Gas.
From www.dreamstime.com
Traditional Tungsten Light Bulb Isolated on White Stock Photo Image Tungsten Light Bulb Gas the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. . Tungsten Light Bulb Gas.
From rt052066.en.made-in-china.com
Tungsten Lamp St46 Carbon Filament Bulb High Performance Edison Style Tungsten Light Bulb Gas the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. the most common noble gases used in light bulbs are xenon, krypton, and argon. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. a halogen. Tungsten Light Bulb Gas.
From pinoyeshop.com
E27 110130v Edison Tungsten Filament Vintage Light Bulb G80 Threaded Tungsten Light Bulb Gas How a tungsten halogen lamp works: a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint. . Tungsten Light Bulb Gas.
From www.homedepot.ca
Globe Electric 01320 60W Vintage Edison G30 Vanity Tungsten Tungsten Light Bulb Gas Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint. the most common noble gases used in light bulbs are xenon, krypton, and argon. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. a mix of halogen gas, nitrogen, and argon maintains high. Tungsten Light Bulb Gas.
From www.alamy.com
Tungsten light bulb isolated on white background Stock Photo Alamy Tungsten Light Bulb Gas as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. How a tungsten halogen lamp works: the only way. Tungsten Light Bulb Gas.
From www.animalia-life.club
Tungsten Light Bulb Filament Tungsten Light Bulb Gas How a tungsten halogen lamp works: a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. Modern. Tungsten Light Bulb Gas.
From alexnld.com
B22 60W Incandescent Bulb 110/220V G125 Edison Tungsten Light Bulb Tungsten Light Bulb Gas 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. How a tungsten halogen lamp works: the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has. Tungsten Light Bulb Gas.
From www.artnews.com
Best Tungsten Light Bulbs Tungsten Light Bulb Gas a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. a mix of halogen gas, nitrogen, and. Tungsten Light Bulb Gas.
From www.alamy.com
Tungsten light bulb lit on black background Stock Photo Alamy Tungsten Light Bulb Gas a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. the most common noble gases used in light bulbs are xenon, krypton, and argon. Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint. How a tungsten halogen lamp works: a halogen lamp. Tungsten Light Bulb Gas.
From www.alamy.com
lighted tungsten light bulb Stock Photo Alamy Tungsten Light Bulb Gas the only way you can get a tungsten bulb to emit a line spectrum is to accompany the filament with a pair of. a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint.. Tungsten Light Bulb Gas.
From www.alamy.com
Tungsten Light Bulb Stock Photo Alamy Tungsten Light Bulb Gas a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. 1) tungsten atoms (yellow) vaporize from the filament and would normally deposit on the inside of. a mix of halogen gas, nitrogen, and argon maintains high pressure inside the bulb, reducing tungsten evaporation. How a tungsten halogen lamp works: Modern. Tungsten Light Bulb Gas.
From www.artnews.com
Best Tungsten Light Bulbs Tungsten Light Bulb Gas the most common noble gases used in light bulbs are xenon, krypton, and argon. Modern halogen lamps often use bromine, which is colorless, instead of iodine, which has a purplish tint. as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. 1) tungsten atoms (yellow) vaporize from the filament and would normally. Tungsten Light Bulb Gas.
From alexnld.com
B22 60W Incandescent Bulb 110/220V G125 Edison Tungsten Light Bulb Tungsten Light Bulb Gas as the filament burns, tungsten particles separate from the filament, eventually causing the filament to weaken. a halogen lamp also uses a tungsten filament, but it is encased inside a much smaller quartz envelope. How a tungsten halogen lamp works: the only way you can get a tungsten bulb to emit a line spectrum is to accompany. Tungsten Light Bulb Gas.