Creutzfeldt-Jakob Disease Botox . Fda approval of botox® cosmetic for temporary. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Today, allergan aesthetics, an abbvie company (nyse: Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. It belongs to a group of human and animal diseases known as.
from healthtian.com
It belongs to a group of human and animal diseases known as. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Today, allergan aesthetics, an abbvie company (nyse: Fda approval of botox® cosmetic for temporary.
Creutzfeldtjakob Disease Causes and Symptoms Healthtian
Creutzfeldt-Jakob Disease Botox Today, allergan aesthetics, an abbvie company (nyse: Today, allergan aesthetics, an abbvie company (nyse: It belongs to a group of human and animal diseases known as. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Fda approval of botox® cosmetic for temporary. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected.
From www.drjimcollins.com
What is CreutzfeldtJakob Disease? Dr. Jim Collins Creutzfeldt-Jakob Disease Botox Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Fda approval of botox® cosmetic for temporary. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Today, allergan aesthetics, an abbvie company (nyse:. Creutzfeldt-Jakob Disease Botox.
From healthtian.com
Creutzfeldtjakob Disease Causes and Symptoms Healthtian Creutzfeldt-Jakob Disease Botox Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. It belongs to a group of human and animal diseases known as. Today, allergan. Creutzfeldt-Jakob Disease Botox.
From www.thelancet.com
Variant or sporadic CreutzfeldtJakob disease? The Lancet Creutzfeldt-Jakob Disease Botox Fda approval of botox® cosmetic for temporary. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Today, allergan aesthetics, an abbvie company (nyse: Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. It belongs to a group of human and animal. Creutzfeldt-Jakob Disease Botox.
From bfhd.wa.gov
CJD CreutzfeldtJakob disease Benton Franklin Health District Creutzfeldt-Jakob Disease Botox It belongs to a group of human and animal diseases known as. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Today, allergan. Creutzfeldt-Jakob Disease Botox.
From medizzy.com
CreutzfeldtJakob MEDizzy Creutzfeldt-Jakob Disease Botox Fda approval of botox® cosmetic for temporary. Today, allergan aesthetics, an abbvie company (nyse: Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. It belongs to a group of human and animal diseases known as. Autopsy series in two cjd surveillance centres. Creutzfeldt-Jakob Disease Botox.
From sciencephotogallery.com
CreutzfeldtJakob disease, MRI scan 1 by Science Photo Library Creutzfeldt-Jakob Disease Botox Fda approval of botox® cosmetic for temporary. Today, allergan aesthetics, an abbvie company (nyse: Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected.. Creutzfeldt-Jakob Disease Botox.
From www.sciencephoto.com
CreutzfeldtJakob disease, CT scan Stock Image C055/6182 Science Creutzfeldt-Jakob Disease Botox Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Today, allergan aesthetics, an abbvie company (nyse: Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Fda approval of botox® cosmetic for temporary.. Creutzfeldt-Jakob Disease Botox.
From www.thelancet.com
Biomarkers and diagnostic guidelines for sporadic CreutzfeldtJakob Creutzfeldt-Jakob Disease Botox Fda approval of botox® cosmetic for temporary. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Today, allergan aesthetics, an abbvie company (nyse:. Creutzfeldt-Jakob Disease Botox.
From www.huffingtonpost.com
2 Patients At VA Hospital In Connecticut May Have Also Been Exposed To Creutzfeldt-Jakob Disease Botox Fda approval of botox® cosmetic for temporary. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. It belongs to a group of human and animal diseases known as. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Today, allergan aesthetics, an. Creutzfeldt-Jakob Disease Botox.
From edition.cnn.com
Case of CreutzfeldtJakob disease confirmed in New Hampshire CNN Creutzfeldt-Jakob Disease Botox Fda approval of botox® cosmetic for temporary. Today, allergan aesthetics, an abbvie company (nyse: Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. It belongs to a group of human and animal diseases known as. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Autopsy series in two cjd surveillance centres. Creutzfeldt-Jakob Disease Botox.
From pixels.com
Brain In Creutzfeldtjakob Disease Photograph by Zephyr Creutzfeldt-Jakob Disease Botox Fda approval of botox® cosmetic for temporary. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. It belongs to a group of human and animal diseases known as. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Cosmetic (onabotulinumtoxina) receives fda. Creutzfeldt-Jakob Disease Botox.
From www.viviennebalonwu.com
CreutzfeldtJakob Disease, CJD Health And Medical Information Creutzfeldt-Jakob Disease Botox It belongs to a group of human and animal diseases known as. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Fda approval of botox® cosmetic for temporary. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Based on effective donor screening and product manufacturing processes, it carries an extremely. Creutzfeldt-Jakob Disease Botox.
From radiologykey.com
CreutzfeldtJakob Disease Radiology Key Creutzfeldt-Jakob Disease Botox Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Fda approval of botox® cosmetic for temporary. Today, allergan aesthetics, an abbvie company (nyse: Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of.. Creutzfeldt-Jakob Disease Botox.
From www.pinterest.com
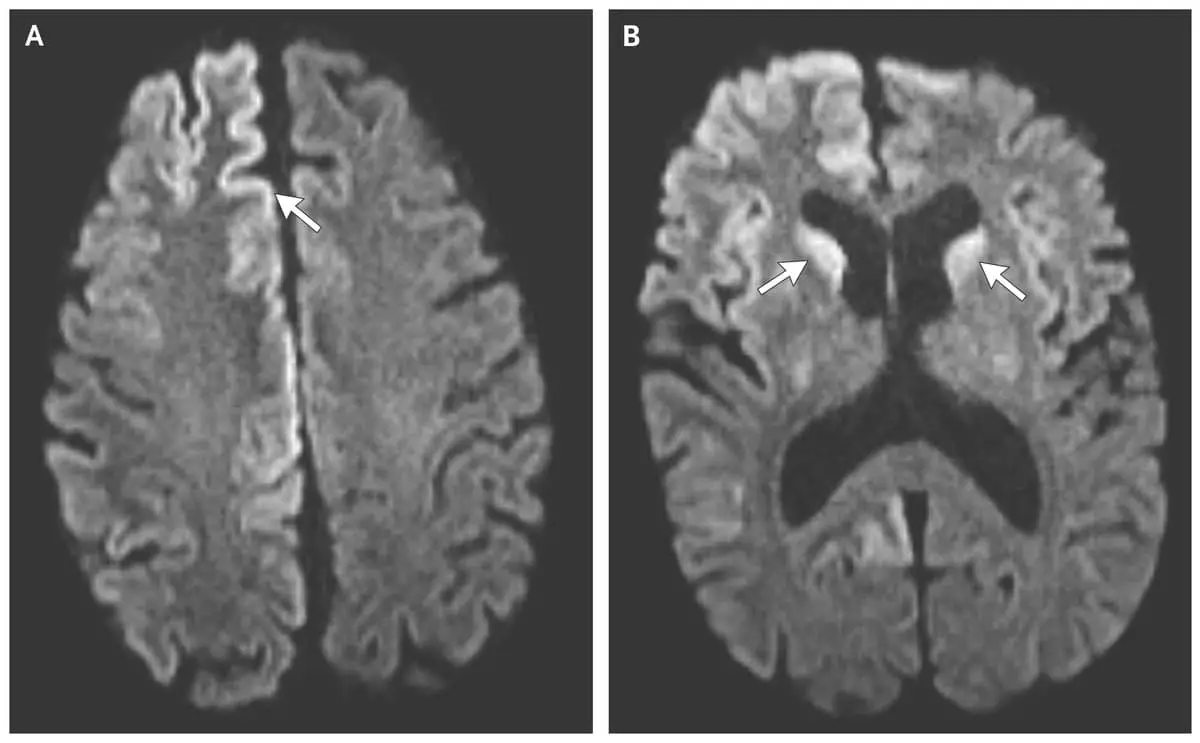
CreutzfeldtJakob disease (CJD) (A & B) Sporadic CJD showing Creutzfeldt-Jakob Disease Botox It belongs to a group of human and animal diseases known as. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Fda approval of botox® cosmetic for temporary. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149. Creutzfeldt-Jakob Disease Botox.
From www.alamy.de
CREUTZFELDTJAKOBKRANKHEIT, MRT Stockfotografie Alamy Creutzfeldt-Jakob Disease Botox Fda approval of botox® cosmetic for temporary. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Today, allergan aesthetics, an abbvie company (nyse: Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. It belongs to a group of human and animal diseases known as. Based on effective donor screening and. Creutzfeldt-Jakob Disease Botox.
From psicologiaymente.com
Enfermedad de CreutzfeldtJakob (ECJ) causas y síntomas Creutzfeldt-Jakob Disease Botox Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Fda approval of botox® cosmetic for temporary. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. It belongs to a group of human and animal diseases known as. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149. Creutzfeldt-Jakob Disease Botox.
From creutzfelts-jakobdisease.weebly.com
CreutzfeldtJakob Disease Creutzfeldt-Jakob Disease Botox Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Fda approval of botox® cosmetic for temporary. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Today, allergan aesthetics, an abbvie company (nyse:. Creutzfeldt-Jakob Disease Botox.
From stellarcaresd.com
CreutzfeldtJakob Disease What Is It? Stellar Care Creutzfeldt-Jakob Disease Botox Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. It belongs to a group of human and animal diseases known as. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Fda approval. Creutzfeldt-Jakob Disease Botox.
From medicinabasica.com
enfermedad de CreutzfeldtJakob Medicina Básica Creutzfeldt-Jakob Disease Botox Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. It belongs to a group of human and animal diseases known as. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Today, allergan. Creutzfeldt-Jakob Disease Botox.
From www.semanticscholar.org
Figure 12 from CreutzfeldtJakob disease. Semantic Scholar Creutzfeldt-Jakob Disease Botox Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. It belongs to a group of human and animal diseases known as. Today, allergan aesthetics, an abbvie company (nyse: Fda approval of botox® cosmetic for temporary. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Autopsy series in two cjd surveillance centres. Creutzfeldt-Jakob Disease Botox.
From neurosaludmunay.com
Enfermedad de Creutzfeldt Jakob Esporádico Presentación del Primer Creutzfeldt-Jakob Disease Botox It belongs to a group of human and animal diseases known as. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Fda approval of botox® cosmetic for temporary. Today, allergan aesthetics, an abbvie company (nyse: Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Autopsy series in two cjd surveillance centres. Creutzfeldt-Jakob Disease Botox.
From microbe-canvas.com
CreutzfeldtJakob Disease Creutzfeldt-Jakob Disease Botox Fda approval of botox® cosmetic for temporary. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. It belongs to a group of human and animal diseases known as. Cosmetic (onabotulinumtoxina) receives fda. Creutzfeldt-Jakob Disease Botox.
From onlinelibrary.wiley.com
Creutzfeldt‐Jakob disease Iwasaki 2017 Neuropathology Wiley Creutzfeldt-Jakob Disease Botox Today, allergan aesthetics, an abbvie company (nyse: Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. It belongs to a group of human and animal diseases known as. Fda approval of botox® cosmetic for temporary. Based on effective donor screening and. Creutzfeldt-Jakob Disease Botox.
From www.medlink.com
CreutzfeldtJakob disease (CJD) MedLink Neurology Creutzfeldt-Jakob Disease Botox It belongs to a group of human and animal diseases known as. Today, allergan aesthetics, an abbvie company (nyse: Fda approval of botox® cosmetic for temporary. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk. Creutzfeldt-Jakob Disease Botox.
From www.medscape.com
New CreutzfeldtJakob Diagnostic Test '100' Accurate Creutzfeldt-Jakob Disease Botox Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. It belongs to a group of human and animal diseases known as. Today, allergan. Creutzfeldt-Jakob Disease Botox.
From www.dovemed.com
CreutzfeldtJakob Disease (CJD) Creutzfeldt-Jakob Disease Botox Today, allergan aesthetics, an abbvie company (nyse: Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Fda approval of botox® cosmetic for temporary. Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. It belongs to a group of human and animal diseases known as. Autopsy series in two cjd surveillance centres. Creutzfeldt-Jakob Disease Botox.
From www.nejm.org
Laboratory Diagnosis of CreutzfeldtJakob Disease NEJM Creutzfeldt-Jakob Disease Botox Today, allergan aesthetics, an abbvie company (nyse: It belongs to a group of human and animal diseases known as. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Fda approval of botox®. Creutzfeldt-Jakob Disease Botox.
From www.youtube.com
What is CJD? CreutzfeldtJakob Disease Prions YouTube Creutzfeldt-Jakob Disease Botox Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. It belongs to a group of human and animal diseases known as. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Fda approval of botox® cosmetic for temporary. Today, allergan aesthetics, an abbvie company (nyse: Based on effective donor screening and. Creutzfeldt-Jakob Disease Botox.
From www.cureus.com
Cureus Imaging Manifestations of CreutzfeldtJakob Disease and Case Creutzfeldt-Jakob Disease Botox It belongs to a group of human and animal diseases known as. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Today, allergan aesthetics, an abbvie company (nyse: Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Cosmetic (onabotulinumtoxina) receives fda. Creutzfeldt-Jakob Disease Botox.
From www.huffingtonpost.com
CreutzfeldtJakob Disease A Look At The Risk Of Contracting The Prion Creutzfeldt-Jakob Disease Botox Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. It belongs to a group of human and animal diseases known as. Fda approval of botox® cosmetic for temporary. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149. Creutzfeldt-Jakob Disease Botox.
From www.scribd.com
CreutzfeldtJakob Disease 1 PDF Human Diseases And Disorders Creutzfeldt-Jakob Disease Botox Today, allergan aesthetics, an abbvie company (nyse: Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Fda approval of botox® cosmetic for temporary. It belongs to a group of human and animal diseases known as. Based on effective donor screening and. Creutzfeldt-Jakob Disease Botox.
From www.pinkybone.com
creutzfeldtjakobdisease (2) PinkyBone Creutzfeldt-Jakob Disease Botox Today, allergan aesthetics, an abbvie company (nyse: Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. It belongs to a group of human and animal diseases known as. Fda approval of botox® cosmetic for temporary. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk. Creutzfeldt-Jakob Disease Botox.
From www.publicdomainfiles.com
Public Domain Picture CreutzfeldtJakob disease photomicrograph ID Creutzfeldt-Jakob Disease Botox Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. Fda approval of botox® cosmetic for temporary. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Today, allergan aesthetics, an abbvie company (nyse:. Creutzfeldt-Jakob Disease Botox.
From radiologykey.com
CreutzfeldtJakob Disease Radiology Key Creutzfeldt-Jakob Disease Botox Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients with clinically suspected. Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Today, allergan aesthetics, an abbvie company (nyse: Cosmetic (onabotulinumtoxina) receives fda approval for moderate to severe. It belongs to a group of human. Creutzfeldt-Jakob Disease Botox.
From www.cureus.com
Cureus Imaging Manifestations of CreutzfeldtJakob Disease and Case Creutzfeldt-Jakob Disease Botox Based on effective donor screening and product manufacturing processes, it carries an extremely remote risk for transmission of. Today, allergan aesthetics, an abbvie company (nyse: It belongs to a group of human and animal diseases known as. Fda approval of botox® cosmetic for temporary. Autopsy series in two cjd surveillance centres revealed that 32% 148 and 47% 149 of patients. Creutzfeldt-Jakob Disease Botox.