What Does M Z Mean . For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Mass/charge ratio is given the symbol m/z (or sometimes m/e). In mass analysis, an electron is taken from molecules to create single charged. M stands for mass and z stands for charge number of ions. An ion with a mass of 56 and a. An ion with a mass of 56 and a. $z$ should not be used in. Mass/charge ratio is given the symbol m/z (or sometimes m/e). M/z represents mass divided by charge number and the horizontal. In mass analysis, an electron is taken from molecules to create single charged. The number of electrons removed is the charge number (for positive ions). M/z represents mass divided by charge number and the horizontal axis in a mass spectrum is expressed in units. The number of electrons removed is the charge number (for positive ions). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M stands for mass and z stands for charge number of ions.
from astrologychart.z33.web.core.windows.net
An ion with a mass of 56 and a. In mass analysis, an electron is taken from molecules to create single charged. The number of electrons removed is the charge number (for positive ions). Mass/charge ratio is given the symbol m/z (or sometimes m/e). M/z represents mass divided by charge number and the horizontal axis in a mass spectrum is expressed in units. M stands for mass and z stands for charge number of ions. An ion with a mass of 56 and a. Mass/charge ratio is given the symbol m/z (or sometimes m/e). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M/z represents mass divided by charge number and the horizontal.
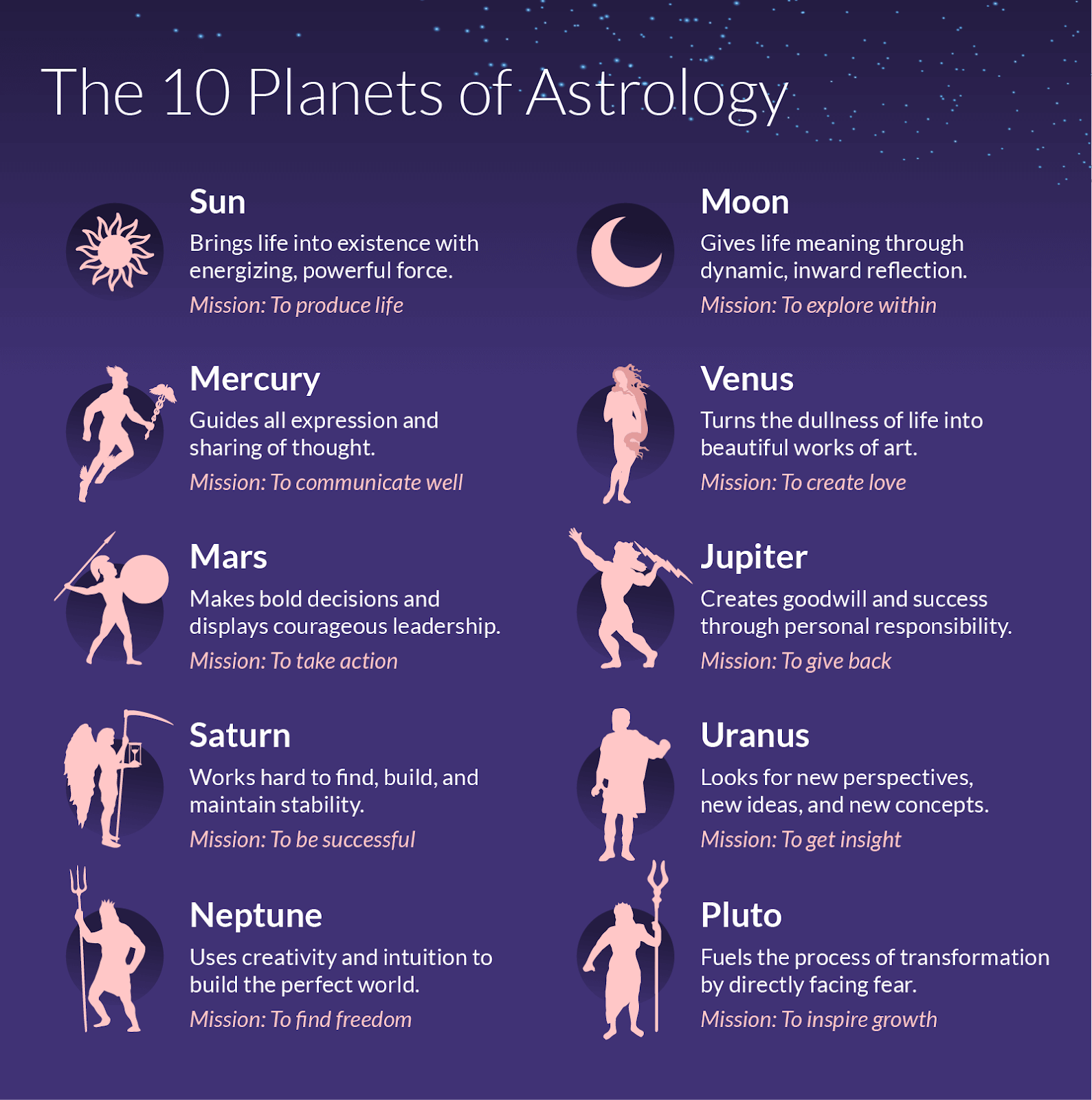
what does each mean in astrology Astrology discover sage goddess
What Does M Z Mean An ion with a mass of 56 and a. M/z represents mass divided by charge number and the horizontal. An ion with a mass of 56 and a. The number of electrons removed is the charge number (for positive ions). $m$ is the atomic mass of the ion (in units ($u$); Mass/charge ratio is given the symbol m/z (or sometimes m/e). In mass analysis, an electron is taken from molecules to create single charged. M stands for mass and z stands for charge number of ions. The number of electrons removed is the charge number (for positive ions). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M/z represents mass divided by charge number and the horizontal axis in a mass spectrum is expressed in units. M stands for mass and z stands for charge number of ions. $z$ should not be used in. Mass/charge ratio is given the symbol m/z (or sometimes m/e). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. An ion with a mass of 56 and a.
From astrologychart.z33.web.core.windows.net
south node calculator astrology What does south node mean in astrology What Does M Z Mean $m$ is the atomic mass of the ion (in units ($u$); Mass/charge ratio is given the symbol m/z (or sometimes m/e). In mass analysis, an electron is taken from molecules to create single charged. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. For example, if an ion. What Does M Z Mean.
From circuitdatablitzkrieg.z21.web.core.windows.net
What Does Low Voltage Mean On An Ecg What Does M Z Mean The number of electrons removed is the charge number (for positive ions). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. In mass analysis, an electron is taken from molecules to create single charged. For example, if an ion had a mass of 28 and a charge of. What Does M Z Mean.
From mechanicmungiuii.z21.web.core.windows.net
Ss Mean In Slang What Does M Z Mean The number of electrons removed is the charge number (for positive ions). M/z represents mass divided by charge number and the horizontal. M stands for mass and z stands for charge number of ions. The number of electrons removed is the charge number (for positive ions). M stands for mass and z stands for charge number of ions. For example,. What Does M Z Mean.
From www.conquerhsc.com
HSC Chemistry Module 8 Inquiry Question 2 What Does M Z Mean For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M/z represents mass divided by charge number and the horizontal. Mass/charge ratio is given the symbol m/z (or. What Does M Z Mean.
From www.youtube.com
mass spectrometry even and odd m/z values YouTube What Does M Z Mean M stands for mass and z stands for charge number of ions. In mass analysis, an electron is taken from molecules to create single charged. In mass analysis, an electron is taken from molecules to create single charged. $z$ should not be used in. Mass/charge ratio is given the symbol m/z (or sometimes m/e). $m$ is the atomic mass of. What Does M Z Mean.
From garagefixaixenbus0a.z21.web.core.windows.net
What Is A 304 Error Code What Does M Z Mean M stands for mass and z stands for charge number of ions. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. In mass analysis, an electron is. What Does M Z Mean.
From learningfullproceed.z21.web.core.windows.net
What Does How Many More Mean In Math Term What Does M Z Mean Mass/charge ratio is given the symbol m/z (or sometimes m/e). $m$ is the atomic mass of the ion (in units ($u$); In mass analysis, an electron is taken from molecules to create single charged. M/z represents mass divided by charge number and the horizontal. The number of electrons removed is the charge number (for positive ions). M stands for mass. What Does M Z Mean.
From astrologychart.z33.web.core.windows.net
what does each mean in astrology Astrology discover sage goddess What Does M Z Mean M/z represents mass divided by charge number and the horizontal. M stands for mass and z stands for charge number of ions. $m$ is the atomic mass of the ion (in units ($u$); The number of electrons removed is the charge number (for positive ions). An ion with a mass of 56 and a. The number of electrons removed is. What Does M Z Mean.
From exonzxqlo.blob.core.windows.net
What Is The Delta Sign In Math at Verla Brooks blog What Does M Z Mean For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. The number of electrons removed is the charge number (for positive ions). M/z represents mass divided by charge. What Does M Z Mean.
From exozgvvac.blob.core.windows.net
What Does M Z Mean In Mass Spectrometry at Gene Corder blog What Does M Z Mean An ion with a mass of 56 and a. An ion with a mass of 56 and a. Mass/charge ratio is given the symbol m/z (or sometimes m/e). M/z represents mass divided by charge number and the horizontal. M stands for mass and z stands for charge number of ions. For example, if an ion had a mass of 28. What Does M Z Mean.
From fixenginepublikuje4w.z21.web.core.windows.net
What Does B Mean On A Car What Does M Z Mean M stands for mass and z stands for charge number of ions. An ion with a mass of 56 and a. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. In mass analysis, an electron is taken from molecules to create single charged. The number of electrons removed. What Does M Z Mean.
From www.msn.com
Andrei Vasilevskiy is approaching a big milestone. What does it mean? What Does M Z Mean The number of electrons removed is the charge number (for positive ions). Mass/charge ratio is given the symbol m/z (or sometimes m/e). M stands for mass and z stands for charge number of ions. M stands for mass and z stands for charge number of ions. Mass/charge ratio is given the symbol m/z (or sometimes m/e). For example, if an. What Does M Z Mean.
From atomicradiichart.z1.web.core.windows.net
what does pm mean in atomic radius Radius periodic ionic across What Does M Z Mean An ion with a mass of 56 and a. $z$ should not be used in. Mass/charge ratio is given the symbol m/z (or sometimes m/e). The number of electrons removed is the charge number (for positive ions). M stands for mass and z stands for charge number of ions. Mass/charge ratio is given the symbol m/z (or sometimes m/e). In. What Does M Z Mean.
From ceaouneo.blob.core.windows.net
What Does D O C T O R Mean In Spanish at Paulette Bayne blog What Does M Z Mean Mass/charge ratio is given the symbol m/z (or sometimes m/e). M/z represents mass divided by charge number and the horizontal. An ion with a mass of 56 and a. $m$ is the atomic mass of the ion (in units ($u$); The number of electrons removed is the charge number (for positive ions). For example, if an ion had a mass. What Does M Z Mean.
From podcasts.apple.com
What does the Budget mean for IFS Zooms In The Economy Apple Podcasts What Does M Z Mean M stands for mass and z stands for charge number of ions. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M/z represents mass divided by charge number and the horizontal. The number of electrons removed is the charge number (for positive ions). $m$ is the atomic mass. What Does M Z Mean.
From dariusz.wieckiewicz.org
Apple discontinues MacBook Air with M1 Chip, but what does that mean What Does M Z Mean $z$ should not be used in. Mass/charge ratio is given the symbol m/z (or sometimes m/e). The number of electrons removed is the charge number (for positive ions). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. $m$ is the atomic mass of the ion (in units ($u$);. What Does M Z Mean.
From dxorolkgv.blob.core.windows.net
What Does Hat Symbol Mean In Math at Christina Medina blog What Does M Z Mean M stands for mass and z stands for charge number of ions. Mass/charge ratio is given the symbol m/z (or sometimes m/e). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. The number of electrons removed is the charge number (for positive ions). An ion with a mass. What Does M Z Mean.
From mechanicbabyxtickoe6y.z21.web.core.windows.net
What Does 2x4 Mean Truck What Does M Z Mean The number of electrons removed is the charge number (for positive ions). The number of electrons removed is the charge number (for positive ions). M/z represents mass divided by charge number and the horizontal axis in a mass spectrum is expressed in units. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge. What Does M Z Mean.
From mechanicskvarillq.z19.web.core.windows.net
Tcs What Does It Mean What Does M Z Mean In mass analysis, an electron is taken from molecules to create single charged. M/z represents mass divided by charge number and the horizontal axis in a mass spectrum is expressed in units. $m$ is the atomic mass of the ion (in units ($u$); For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge. What Does M Z Mean.
From mechanictambussab9.z21.web.core.windows.net
What Does Obs Mean In Ford Trucks What Does M Z Mean $m$ is the atomic mass of the ion (in units ($u$); $z$ should not be used in. An ion with a mass of 56 and a. M/z represents mass divided by charge number and the horizontal. Mass/charge ratio is given the symbol m/z (or sometimes m/e). The number of electrons removed is the charge number (for positive ions). For example,. What Does M Z Mean.
From repairmachinekleparyv3.z21.web.core.windows.net
What Does It Mean When A Car Is Cammed What Does M Z Mean An ion with a mass of 56 and a. In mass analysis, an electron is taken from molecules to create single charged. M stands for mass and z stands for charge number of ions. Mass/charge ratio is given the symbol m/z (or sometimes m/e). For example, if an ion had a mass of 28 and a charge of 1+, its. What Does M Z Mean.
From repairfixaltefluga5g.z21.web.core.windows.net
What Does Tpm Mean On A Computer What Does M Z Mean $m$ is the atomic mass of the ion (in units ($u$); For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M stands for mass and z stands for charge number of ions. An ion with a mass of 56 and a. M stands for mass and z stands. What Does M Z Mean.
From atomicradiichart.z1.web.core.windows.net
what does pm mean in atomic radius Radius periodic ionic across What Does M Z Mean In mass analysis, an electron is taken from molecules to create single charged. In mass analysis, an electron is taken from molecules to create single charged. $m$ is the atomic mass of the ion (in units ($u$); An ion with a mass of 56 and a. For example, if an ion had a mass of 28 and a charge of. What Does M Z Mean.
From atomicradiichart.z1.web.core.windows.net
what does pm mean in atomic radius Radius periodic ionic across What Does M Z Mean For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. $m$ is the atomic mass of the ion (in units ($u$); In mass analysis, an electron is taken from molecules to create single charged. M/z represents mass divided by charge number and the horizontal. Mass/charge ratio is given the. What Does M Z Mean.
From ar.inspiredpencil.com
Z Score Table Calculator What Does M Z Mean For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. An ion with a mass of 56 and a. In mass analysis, an electron is taken from molecules to create single charged. M/z represents mass divided by charge number and the horizontal. For example, if an ion had a. What Does M Z Mean.
From enginemalostiemposm380.z21.web.core.windows.net
What Does Check Engine Light Flashing Mean What Does M Z Mean $m$ is the atomic mass of the ion (in units ($u$); $z$ should not be used in. The number of electrons removed is the charge number (for positive ions). Mass/charge ratio is given the symbol m/z (or sometimes m/e). Mass/charge ratio is given the symbol m/z (or sometimes m/e). An ion with a mass of 56 and a. M/z represents. What Does M Z Mean.
From enginerileysequitur.z21.web.core.windows.net
What Do The Lines On Resistors Mean What Does M Z Mean An ion with a mass of 56 and a. M stands for mass and z stands for charge number of ions. The number of electrons removed is the charge number (for positive ions). In mass analysis, an electron is taken from molecules to create single charged. In mass analysis, an electron is taken from molecules to create single charged. M. What Does M Z Mean.
From astrologychart.z33.web.core.windows.net
what does each mean in astrology Astrology discover sage goddess What Does M Z Mean In mass analysis, an electron is taken from molecules to create single charged. An ion with a mass of 56 and a. Mass/charge ratio is given the symbol m/z (or sometimes m/e). M/z represents mass divided by charge number and the horizontal. $m$ is the atomic mass of the ion (in units ($u$); M/z represents mass divided by charge number. What Does M Z Mean.
From dariusz.wieckiewicz.org
Apple discontinues MacBook Air with M1 Chip, but what does that mean What Does M Z Mean M/z represents mass divided by charge number and the horizontal axis in a mass spectrum is expressed in units. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. Mass/charge ratio is given the symbol m/z (or sometimes m/e). In mass analysis, an electron is taken from molecules to. What Does M Z Mean.
From mechanicmungiuii.z21.web.core.windows.net
Ss Mean In Slang What Does M Z Mean In mass analysis, an electron is taken from molecules to create single charged. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M/z represents mass divided by charge number and the horizontal axis in a mass spectrum is expressed in units. Mass/charge ratio is given the symbol m/z. What Does M Z Mean.
From learningfullproceed.z21.web.core.windows.net
What Does How Many More Mean In Math Term What Does M Z Mean An ion with a mass of 56 and a. M stands for mass and z stands for charge number of ions. $z$ should not be used in. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. For example, if an ion had a mass of 28 and a. What Does M Z Mean.
From fixenginepublikuje4w.z21.web.core.windows.net
What Does S Mean On A Car What Does M Z Mean Mass/charge ratio is given the symbol m/z (or sometimes m/e). The number of electrons removed is the charge number (for positive ions). For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. M stands for mass and z stands for charge number of ions. For example, if an ion. What Does M Z Mean.
From fixenginepublikuje4w.z21.web.core.windows.net
What Does Service 4wd Mean On Jeep Grand Cherokee What Does M Z Mean M stands for mass and z stands for charge number of ions. M/z represents mass divided by charge number and the horizontal. In mass analysis, an electron is taken from molecules to create single charged. Mass/charge ratio is given the symbol m/z (or sometimes m/e). $m$ is the atomic mass of the ion (in units ($u$); For example, if an. What Does M Z Mean.
From dariusz.wieckiewicz.org
Apple discontinues MacBook Air with M1 Chip, but what does that mean What Does M Z Mean For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. In mass analysis, an electron is taken from molecules to create single charged. M stands for mass and z stands for charge number of ions. The number of electrons removed is the charge number (for positive ions). For example,. What Does M Z Mean.
From giolvagox.blob.core.windows.net
What Is Z Score In Growth Chart at Rhonda Holbrook blog What Does M Z Mean An ion with a mass of 56 and a. Mass/charge ratio is given the symbol m/z (or sometimes m/e). M stands for mass and z stands for charge number of ions. For example, if an ion had a mass of 28 and a charge of 1+, its mass/charge ratio would be 28. For example, if an ion had a mass. What Does M Z Mean.