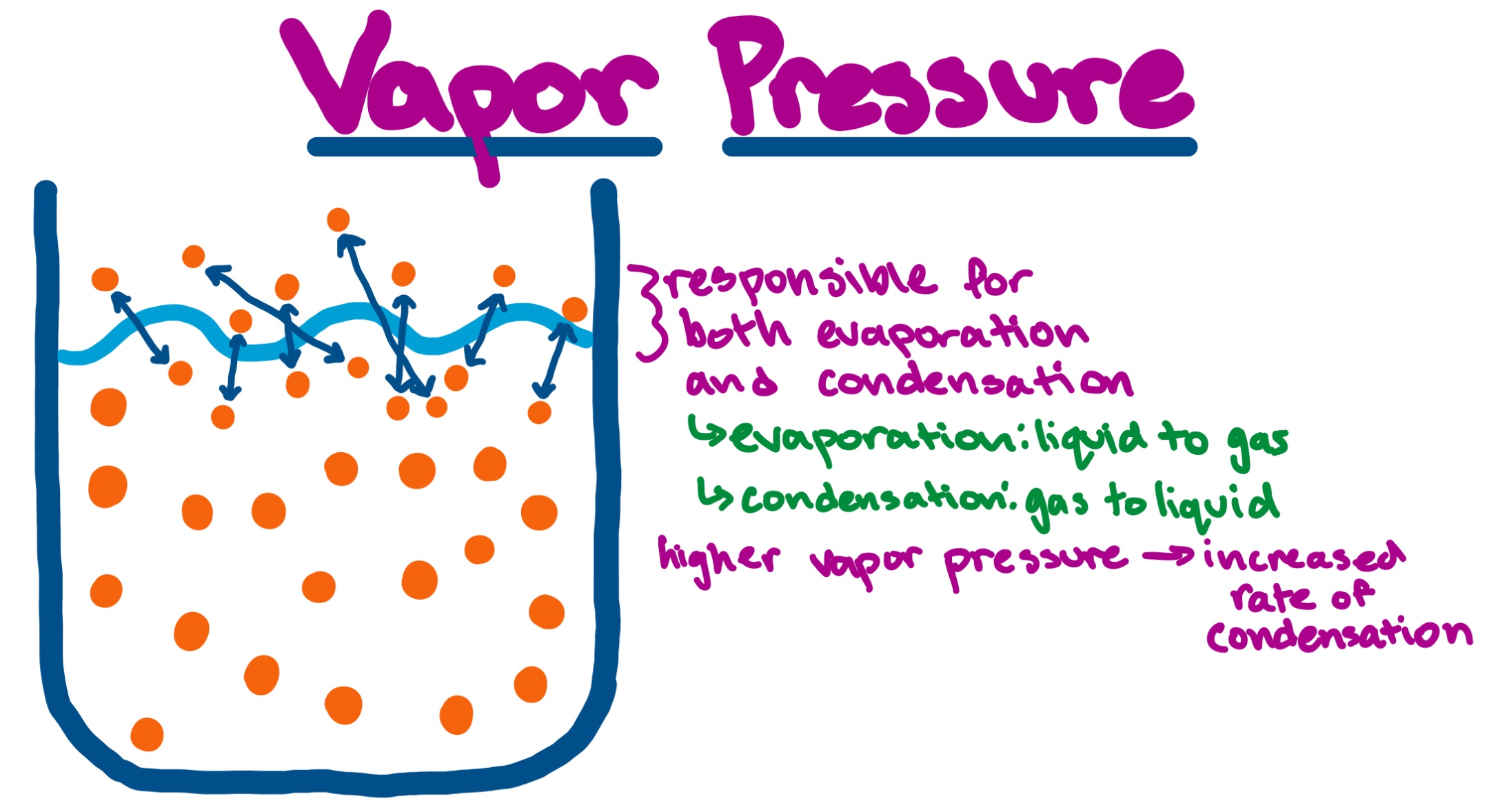
What Is Vapor Pressure . Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure varies with. Vapor pressure is the pressure exerted by a vapor in dynamic equilibrium with a liquid. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and boiling point. Learn how vapour pressure increases with. Learn how vapor pressure depends on. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form.
from
Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Vapor pressure is the pressure exerted by a vapor in dynamic equilibrium with a liquid. Learn how vapor pressure depends on. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and boiling point. Learn how vapour pressure increases with. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Learn how vapor pressure varies with.
What Is Vapor Pressure Learn how vapor pressure varies with. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Vapor pressure is the pressure exerted by a vapor in dynamic equilibrium with a liquid. Learn how vapor pressure varies with. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and boiling point. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn how vapour pressure increases with. Learn how vapor pressure depends on.
From www.slideserve.com
PPT Evaporation, Vapor Pressure, and Intermolecular Forces PowerPoint What Is Vapor Pressure Learn how vapor pressure varies with. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and boiling point. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapor pressure depends on. Learn how vapor pressure depends on. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn how vapor pressure varies with. Learn how vapour pressure increases with. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapor pressure depends on. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Vapor pressure is the equilibrium pressure of a vapor above its liquid. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Learn how vapor pressure depends on. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Learn how vapour pressure increases with. Vapor pressure is the equilibrium pressure of a vapor above its. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Learn how vapor pressure depends on. Learn how vapor pressure varies with. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and. What Is Vapor Pressure.
From amariqoleon.blogspot.com
What is Vapor Pressure AmariqoLeon What Is Vapor Pressure Learn how vapour pressure increases with. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and boiling point. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Vapor pressure is the pressure exerted by a vapor in dynamic equilibrium with a liquid. Learn the. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapor pressure depends on. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed. What Is Vapor Pressure.
From mrlvohyvol.blogspot.com
How To Find Vapor Pressure Of A Solution Determine vapor pressure of What Is Vapor Pressure Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Learn how vapour pressure increases with. Learn how vapor pressure depends on. Vapor pressure is the partial pressure of a substance in the gas phase that exists above. What Is Vapor Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure Learn how vapor pressure depends on. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapor pressure varies with. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn how vapour pressure increases with. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Learn how vapor pressure depends on. Vapor pressure is the pressure exerted by a vapor in dynamic equilibrium. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Learn how vapor pressure varies with. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapour pressure increases with. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and boiling point. Learn how vapor pressure depends on. Vapor pressure is the pressure exerted by a vapor in dynamic. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapour pressure increases with. Vapor pressure is the pressure exerted by a vapor in dynamic equilibrium with a liquid. Vapor pressure. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapour pressure increases with. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Vapor pressure is the partial. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn how vapour pressure increases with. Learn how vapor pressure depends on. Learn how vapor pressure varies with. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapour pressure increases with. Learn how vapor pressure varies with. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Vapor pressure is the pressure exerted by a vapor in dynamic equilibrium with a liquid. Learn how vapor pressure depends on. Learn how. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and boiling point. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its. What Is Vapor Pressure.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation, free download ID352441 What Is Vapor Pressure Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn how vapour pressure increases with. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure varies with. Learn how vapor pressure depends on. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapour pressure increases with. Learn what vapour pressure. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapour pressure increases with. Learn how vapor pressure varies with. Learn the definition, characteristics and applications. What Is Vapor Pressure.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download What Is Vapor Pressure Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container.. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Vapor pressure is the pressure exerted by a vapor in dynamic equilibrium with a liquid. Learn how vapor pressure varies with. Learn how vapor pressure depends on. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container.. What Is Vapor Pressure.
From www.youtube.com
Which Compound Has a Higher Vapor Pressure? Intermolecular Force What Is Vapor Pressure Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and boiling point. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapour pressure increases with. Learn how vapor pressure varies with. Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and boiling point. Learn the definition, characteristics and applications of vapor pressure, the pressure. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapor pressure depends on. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed container. Learn what vapour pressure is, how it depends on temperature and nature. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Vapor pressure is the pressure exerted by a vapor in dynamic equilibrium with a liquid. Learn what vapour pressure is, how it depends on temperature. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapor pressure varies with. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn how vapour pressure increases with. Learn how vapor pressure depends on. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed. What Is Vapor Pressure.
From
What Is Vapor Pressure Learn how vapor pressure depends on temperature, intermolecular forces, and enthalpy of. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn how vapor pressure varies with. Vapor pressure is the partial pressure of a substance in the gas phase that exists above a sample of the liquid in a closed. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Vapor pressure is the equilibrium pressure of a vapor above its liquid or solid in a closed container. Learn how vapor pressure depends on. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed. What Is Vapor Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and boiling point. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure depends on. Vapor pressure is the partial pressure of a substance. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapor pressure is the pressure exerted by a vapor in dynamic equilibrium with a liquid. Learn what vapour pressure is, how it depends on temperature and nature of the liquid, and how it relates to raoult's law and boiling point. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases.. What Is Vapor Pressure.
From
What Is Vapor Pressure Vapor pressure is the pressure exerted by a vapor in dynamic equilibrium with a liquid. Vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn the definition, characteristics and applications of vapor pressure, the pressure of a vapor in equilibrium with its condensed phases. Learn how vapor pressure depends on temperature, intermolecular. What Is Vapor Pressure.