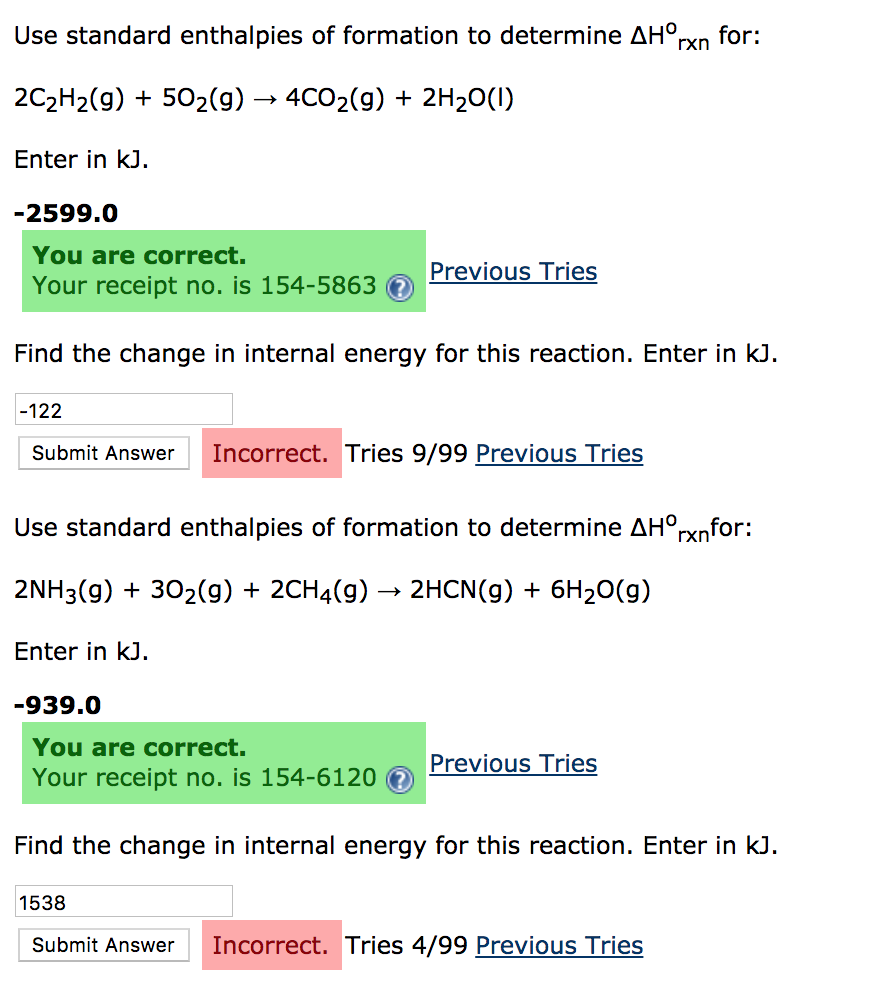
Use Standard Enthalpies Of Formation To Calculate . the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2 (g).
from www.chegg.com
the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2 (g).
Solved Use standard enthalpies of formation to determine
Use Standard Enthalpies Of Formation To Calculate however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2 (g). the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent.
From www.coursehero.com
[Solved] Using the table of standard formation enthalpies that you'll Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance. Use Standard Enthalpies Of Formation To Calculate.
From pafjpegeuw.blogspot.com
How To Calculate Standard Enthalpy Change Using Heats Of Formation Use Standard Enthalpies Of Formation To Calculate however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. Use Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVED Calculate the standard Gibbs energy of the reaction CO(g Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. the standard enthalpy of formation is the enthalpy change when 1 mol. Use Standard Enthalpies Of Formation To Calculate.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Use Standard Enthalpies Of Formation To Calculate however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. . Use Standard Enthalpies Of Formation To Calculate.
From www.chegg.com
Solved Part A Use standard enthalpies of formation to Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2 (g). the standard enthalpy of formation, δh of, is the enthalpy change for. Use Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVEDUse the standard enthalpies of formation available here to Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance. Use Standard Enthalpies Of Formation To Calculate.
From solvedlib.com
Using the standard molar enthalpies of formation give… SolvedLib Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2 (g). the standard enthalpy of formation, also known as the heat of formation, is the enthalpy. Use Standard Enthalpies Of Formation To Calculate.
From printablelibmolines.z13.web.core.windows.net
How To Find Enthalpy In Chemistry Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. . Use Standard Enthalpies Of Formation To Calculate.
From kunduz.com
[ANSWERED] Use the standard enthalpies of formation to calculate the AH Use Standard Enthalpies Of Formation To Calculate however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. using. Use Standard Enthalpies Of Formation To Calculate.
From www.slideshare.net
Calculations using standard enthalpies of formation Use Standard Enthalpies Of Formation To Calculate however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. using. Use Standard Enthalpies Of Formation To Calculate.
From brightideas.houstontx.gov
Using The Standard Enthalpies Of Formations Find The Standard Enthalpy Use Standard Enthalpies Of Formation To Calculate however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of. Use Standard Enthalpies Of Formation To Calculate.
From www.vrogue.co
Standard Enthalpy Of Formation And Standard Free Ener vrogue.co Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the. Use Standard Enthalpies Of Formation To Calculate.
From www.vrogue.co
Solved Using The Table Of Standard Formation Enthalpi vrogue.co Use Standard Enthalpies Of Formation To Calculate using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2 (g). the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the. Use Standard Enthalpies Of Formation To Calculate.
From www.vrogue.co
9 Calculate The Enthalpy For The Reaction Shown Below vrogue.co Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2 (g). the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the. Use Standard Enthalpies Of Formation To Calculate.
From printablelibmolines.z13.web.core.windows.net
How To Find Standard Enthalpy Of Reaction Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. the. Use Standard Enthalpies Of Formation To Calculate.
From study.com
How to Draw & Label Enthalpy Diagrams Lesson Use Standard Enthalpies Of Formation To Calculate however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in. Use Standard Enthalpies Of Formation To Calculate.
From www.bartleby.com
Answered Using the table of standard formation… bartleby Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is the enthalpy. Use Standard Enthalpies Of Formation To Calculate.
From www.toppr.com
Use the given standard enthalpies of formation (in kJ/mol) to determine Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. the standard enthalpy of formation is the enthalpy change when 1 mol. Use Standard Enthalpies Of Formation To Calculate.
From youtube.com
Calculate Reaction Change in Enthalpy, Entropy and Free Energy YouTube Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. using the values in the above table of standard enthalpies of formation, calculate the. Use Standard Enthalpies Of Formation To Calculate.
From www.thesciencehive.co.uk
Lattice Enthalpy* — the science sauce Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation, δh of, is the enthalpy change for. Use Standard Enthalpies Of Formation To Calculate.
From www.slideserve.com
PPT Energetics PowerPoint Presentation ID1204656 Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. . Use Standard Enthalpies Of Formation To Calculate.
From pdfprof.com
enthalpies standard de formation et entropie standard Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a. Use Standard Enthalpies Of Formation To Calculate.
From ar.inspiredpencil.com
Enthalpy Of Reaction Use Standard Enthalpies Of Formation To Calculate using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2 (g). the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the. Use Standard Enthalpies Of Formation To Calculate.
From slideplayer.com
Chapter 5 Thermochemistry ppt download Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2 (g). the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation. Use Standard Enthalpies Of Formation To Calculate.
From centpxdm.blob.core.windows.net
Standard Enthalpy Of Formation Quizlet at Kristin Kremer blog Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of formation is the enthalpy. Use Standard Enthalpies Of Formation To Calculate.
From classzonemailmerge.z22.web.core.windows.net
How To Calculate The Heat Of Formation Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o. Use Standard Enthalpies Of Formation To Calculate.
From www.chegg.com
Solved Use standard enthalpies of formation to calculate Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. using the values in the above table of standard enthalpies of formation, calculate the. Use Standard Enthalpies Of Formation To Calculate.
From www.chegg.com
Solved Use standard enthalpies of formation to calculate Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from. Use Standard Enthalpies Of Formation To Calculate.
From www.chegg.com
Solved Using the table of standard formation enthalpies that Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. however, we can use standard enthalpies of formation,δ hf0, as functional equivalents. Use Standard Enthalpies Of Formation To Calculate.
From www.chegg.com
Solved Use standard enthalpies of formation to determine Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated. Use Standard Enthalpies Of Formation To Calculate.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Use Standard Enthalpies Of Formation To Calculate however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance. Use Standard Enthalpies Of Formation To Calculate.
From www.chegg.com
Solved Part A Use standard enthalpies of formation to Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2 (g). the standard enthalpy of formation is the enthalpy change when 1. Use Standard Enthalpies Of Formation To Calculate.
From www.numerade.com
SOLVED Use standard enthalpies of formation (AH; values) to calculate Use Standard Enthalpies Of Formation To Calculate the standard enthalpy of formation, δh of, is the enthalpy change for a formation equation when all substances are in their standard. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is. Use Standard Enthalpies Of Formation To Calculate.
From oneclass.com
OneClass Part A Use standard enthalpies of formation to calculate the Use Standard Enthalpies Of Formation To Calculate using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2 (g). the standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum. Use Standard Enthalpies Of Formation To Calculate.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Use Standard Enthalpies Of Formation To Calculate however, we can use standard enthalpies of formation,δ hf0, as functional equivalents of a substance’s enthalpy. the standard enthalpy of reaction (\(δh^o_{rxn}\)) can be calculated from the sum of the standard enthalpies of formation. using the values in the above table of standard enthalpies of formation, calculate the δh reaction o for the formation of no 2. Use Standard Enthalpies Of Formation To Calculate.