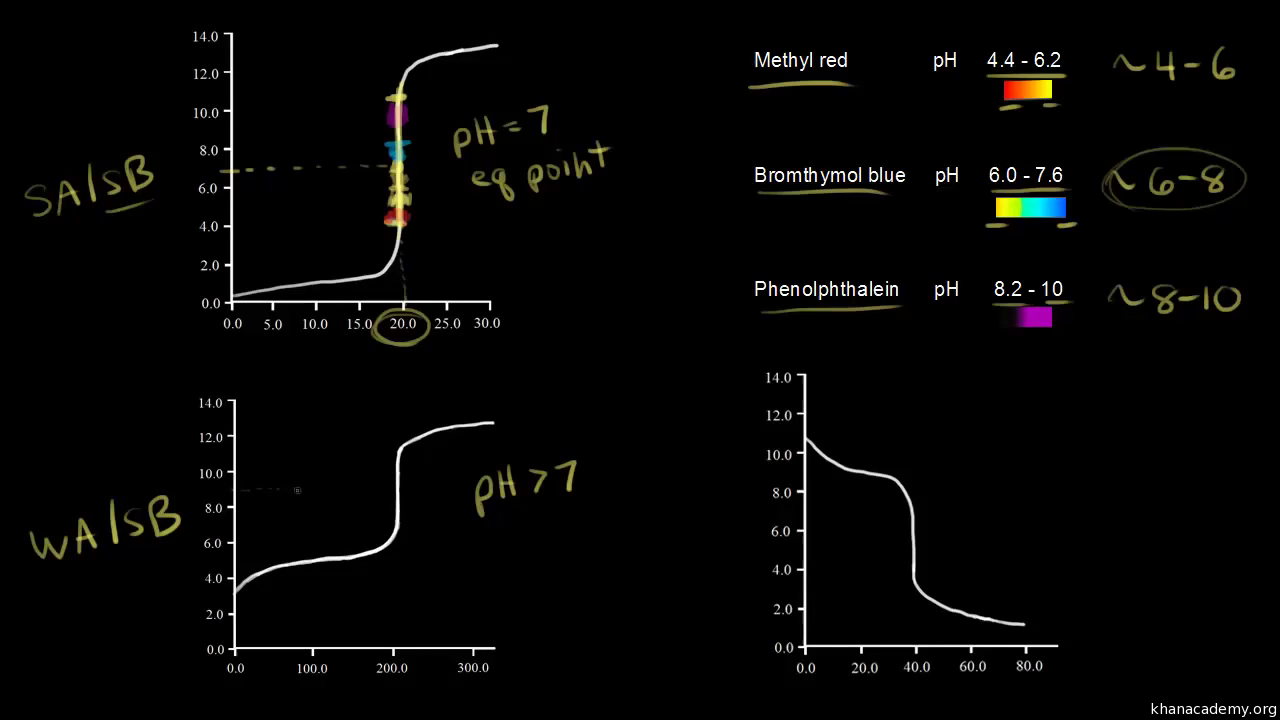
Indicators Are Used To Determine The Equivalence Point . In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically.
from www.reddit.com
the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the.
Equivalence Point and End Point r/Mcat
Indicators Are Used To Determine The Equivalence Point we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Indicators Are Used To Determine The Equivalence Point A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. we have stated that a good indicator should have a pkin value that is close to the expected ph. Indicators Are Used To Determine The Equivalence Point.
From studylib.net
Finding the Equivalence Point (calculation method) • Strong Acid vs Indicators Are Used To Determine The Equivalence Point we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. In other words, while titrating, it. Indicators Are Used To Determine The Equivalence Point.
From www.chegg.com
Solved determine the equivalence point volume, half Indicators Are Used To Determine The Equivalence Point identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point.. Indicators Are Used To Determine The Equivalence Point.
From quizdbmonarchist.z21.web.core.windows.net
How To Find Equivalence Point Indicators Are Used To Determine The Equivalence Point In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. the trick is to identify the ph at the equivalence point (the salt of the compound. Indicators Are Used To Determine The Equivalence Point.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Indicators Are Used To Determine The Equivalence Point identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. the equivalence point of a chemical reaction is the. Indicators Are Used To Determine The Equivalence Point.
From www.coursehero.com
[Solved] Which indicator is best suited to determine the equivalence Indicators Are Used To Determine The Equivalence Point the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. we have stated that a good. Indicators Are Used To Determine The Equivalence Point.
From pediaa.com
Difference Between Equivalence Point and Endpoint Definition Indicators Are Used To Determine The Equivalence Point A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. the trick is to identify the ph at the equivalence point (the salt of the compound. Indicators Are Used To Determine The Equivalence Point.
From www.youtube.com
How to Find the Equivalence Point on a Titration Graph In Excel YouTube Indicators Are Used To Determine The Equivalence Point we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it is a point where the amount of added titrant is. Indicators Are Used To Determine The Equivalence Point.
From slideplayer.com
Chapter 17 Part ppt download Indicators Are Used To Determine The Equivalence Point the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the. Indicators Are Used To Determine The Equivalence Point.
From study.com
Finding the Equivalence Point Titration & Examples Lesson Indicators Are Used To Determine The Equivalence Point the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating). Indicators Are Used To Determine The Equivalence Point.
From the-equivalent.com
How to determine equivalence point The Equivalent Indicators Are Used To Determine The Equivalence Point the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. the equivalence point of a chemical reaction is the. Indicators Are Used To Determine The Equivalence Point.
From www.reddit.com
Equivalence Point and End Point r/Mcat Indicators Are Used To Determine The Equivalence Point the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. the equivalence point of a. Indicators Are Used To Determine The Equivalence Point.
From quizpseudology.z21.web.core.windows.net
How To Find Equivalence Point On Excel Graph Indicators Are Used To Determine The Equivalence Point In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. the equivalence point of a chemical reaction is the point at which. Indicators Are Used To Determine The Equivalence Point.
From www.chegg.com
Solved (c) Which indicator should be used to find the Indicators Are Used To Determine The Equivalence Point identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. A properly picked indicator will change color over a drop, as the line is almost vertical. Indicators Are Used To Determine The Equivalence Point.
From quizspattering.z21.web.core.windows.net
How To Find Equivalence Point On Graph Indicators Are Used To Determine The Equivalence Point the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. we have stated that a good. Indicators Are Used To Determine The Equivalence Point.
From www.differencebetween.com
Difference Between Half Equivalence Point and Equivalence Point Indicators Are Used To Determine The Equivalence Point identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. A properly picked indicator will change color over a drop,. Indicators Are Used To Determine The Equivalence Point.
From www.chegg.com
Solved Determine the equivalence point volume to two decimal Indicators Are Used To Determine The Equivalence Point the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. identifying the equivalence point allows chemists to determine the. Indicators Are Used To Determine The Equivalence Point.
From dxovrotfq.blob.core.windows.net
Titration Of Indicators at Beverly Estrada blog Indicators Are Used To Determine The Equivalence Point identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. the equivalence point of a chemical reaction is the point at which equal quantities of reactants. Indicators Are Used To Determine The Equivalence Point.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Indicators Are Used To Determine The Equivalence Point identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. A properly picked indicator will change color over a drop, as the line is almost vertical at. Indicators Are Used To Determine The Equivalence Point.
From www.vrogue.co
Acid Base Titrations · Chemistry Titration Curves Equivalence Point Indicators Are Used To Determine The Equivalence Point we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. the trick is to identify the ph at the equivalence point (the salt of the. Indicators Are Used To Determine The Equivalence Point.
From exolnazbp.blob.core.windows.net
A Titration Reached The Equivalence Point When 16.1 Ml at Amber Holmes blog Indicators Are Used To Determine The Equivalence Point the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. A properly picked indicator will change color over a drop,. Indicators Are Used To Determine The Equivalence Point.
From educationsupply.z13.web.core.windows.net
How To Determine Equivalence Point Indicators Are Used To Determine The Equivalence Point A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. identifying the equivalence point allows chemists to determine the exact amount of titrant needed to. Indicators Are Used To Determine The Equivalence Point.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Indicators Are Used To Determine The Equivalence Point we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. the equivalence point of a. Indicators Are Used To Determine The Equivalence Point.
From the-equivalent.com
How to find half equivalence point The Equivalent Indicators Are Used To Determine The Equivalence Point identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. we have stated that a good indicator should have. Indicators Are Used To Determine The Equivalence Point.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Indicators Are Used To Determine The Equivalence Point we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. A properly picked indicator will change color over a drop, as the line is almost vertical. Indicators Are Used To Determine The Equivalence Point.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Indicators Are Used To Determine The Equivalence Point we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. A properly picked indicator will change color over a drop, as the line is almost vertical. Indicators Are Used To Determine The Equivalence Point.
From slideplayer.com
Concentration of Solutions ppt download Indicators Are Used To Determine The Equivalence Point identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. we have stated that a good indicator should have. Indicators Are Used To Determine The Equivalence Point.
From www.slideserve.com
PPT Titration Term Review PowerPoint Presentation, free download ID Indicators Are Used To Determine The Equivalence Point A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. the equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the. Indicators Are Used To Determine The Equivalence Point.
From edurev.in
End Point, Equivalence Point and Indicators Physical Chemistry PDF Indicators Are Used To Determine The Equivalence Point we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. the equivalence point of a chemical reaction is the point at which equal quantities of. Indicators Are Used To Determine The Equivalence Point.
From quizspattering.z21.web.core.windows.net
How To Find Equivalence Point Indicators Are Used To Determine The Equivalence Point In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. we have stated that a good indicator should have a pkin value that is close to. Indicators Are Used To Determine The Equivalence Point.
From www.youtube.com
2020 P1 Q15 Determine Suitable Indicator for Equivalence Point YouTube Indicators Are Used To Determine The Equivalence Point A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react. Indicators Are Used To Determine The Equivalence Point.
From www.chegg.com
Solved (a) Which indicator should be used to find the Indicators Are Used To Determine The Equivalence Point A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. the trick is to identify the ph at the equivalence point (the salt of the. Indicators Are Used To Determine The Equivalence Point.
From differencesfinder.com
Difference Between Equivalence Point and End Point Differences Finder Indicators Are Used To Determine The Equivalence Point we have stated that a good indicator should have a pkin value that is close to the expected ph at the equivalence point. the trick is to identify the ph at the equivalence point (the salt of the compound you are titrating) and pick an indicator that changes ph over that range. the equivalence point of a. Indicators Are Used To Determine The Equivalence Point.
From quizpseudology.z21.web.core.windows.net
How To Find Equivalence Point Indicators Are Used To Determine The Equivalence Point identifying the equivalence point allows chemists to determine the exact amount of titrant needed to react completely with the. A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. we have stated that a good indicator should have a pkin value that is close to the expected ph. Indicators Are Used To Determine The Equivalence Point.
From slideplayer.com
Titration A pH meter or indicators are used to determine when the Indicators Are Used To Determine The Equivalence Point In other words, while titrating, it is a point where the amount of added titrant is enough to neutralize the analyte solution completely. A properly picked indicator will change color over a drop, as the line is almost vertical at the equivalence point. the trick is to identify the ph at the equivalence point (the salt of the compound. Indicators Are Used To Determine The Equivalence Point.