Bromine Chlorine Gas Reaction . Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Bromine + chlorine = bromine monochloride. Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. These reactions are usually spontaneous. Bromine is less reactive, means it. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Chlorine, bromine or iodine can be added to an alkene. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample.
from www.numerade.com
Chlorine, bromine or iodine can be added to an alkene. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. These reactions are usually spontaneous. Bromine + chlorine = bromine monochloride. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Bromine is less reactive, means it.
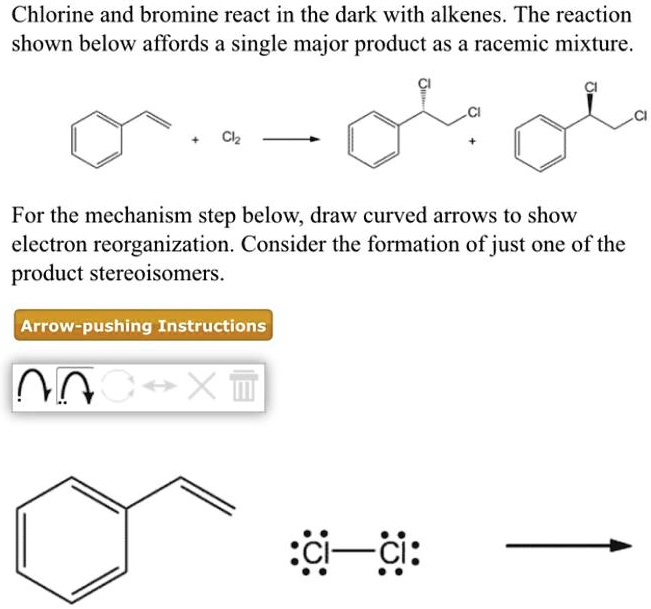
SOLVED Chlorine and bromine react in the dark with alkenes. The reaction shown below affords a
Bromine Chlorine Gas Reaction Chlorine, bromine or iodine can be added to an alkene. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. Bromine is less reactive, means it. Chlorine, bromine or iodine can be added to an alkene. These reactions are usually spontaneous. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Bromine + chlorine = bromine monochloride.
From www.numerade.com
SOLVED Aluminum bromide and chlorine gas react to form aluminum chloride and bromine gas. Bromine Chlorine Gas Reaction The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in. Bromine Chlorine Gas Reaction.
From www.coursehero.com
[Solved] The reaction of bromine gas with chlorine gas, shown here, has a Kc... Course Hero Bromine Chlorine Gas Reaction Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. Chlorine, bromine or iodine can be added to an alkene. These reactions are usually spontaneous. Bromine is less reactive, means it. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Bromine + chlorine = bromine monochloride. When alkenes. Bromine Chlorine Gas Reaction.
From chem.libretexts.org
10.4 Reactions of Alkenes Addition of Bromine and Chlorine to Alkenes Chemistry LibreTexts Bromine Chlorine Gas Reaction When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Bromine is less reactive, means it. Chlorine, bromine or iodine can be added to an alkene. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence. Bromine Chlorine Gas Reaction.
From solvedlib.com
45. The reaction of bromine gas with chlorine gas, sh… SolvedLib Bromine Chlorine Gas Reaction Bromine is less reactive, means it. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. These reactions are usually spontaneous. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Bromine + chlorine = bromine monochloride. Br + cl = brcl. Bromine Chlorine Gas Reaction.
From solvedlib.com
9/The reaction of bromine gas with chlorine gas, show… SolvedLib Bromine Chlorine Gas Reaction Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. These reactions are usually spontaneous. Bromine is less reactive, means it. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The reaction between c=c double bond and bromine (br 2) can be used as a test for the. Bromine Chlorine Gas Reaction.
From studylib.net
Displacement reactions Reactions of chlorine Bromine Chlorine Gas Reaction The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Chlorine, bromine or iodine can be added to an alkene. Bromine + chlorine = bromine monochloride. These reactions are usually spontaneous. Different. Bromine Chlorine Gas Reaction.
From www.coursehero.com
[Solved] 4.7. 3. The reaction of bromine gas with chlorine gas, shown here,... Course Hero Bromine Chlorine Gas Reaction Bromine is less reactive, means it. Chlorine, bromine or iodine can be added to an alkene. Bromine + chlorine = bromine monochloride. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. These reactions are usually spontaneous. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent. Bromine Chlorine Gas Reaction.
From www.sciencephoto.com
Displacement reaction of bromine gas Stock Image A500/0201 Science Photo Library Bromine Chlorine Gas Reaction Bromine + chlorine = bromine monochloride. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. Chlorine, bromine or iodine can be added to an alkene. Br + cl = brcl is a synthesis reaction where one mole of. Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED For the following reaction; 67.3 grams of bromine are allowed to react with 28.1 grams Bromine Chlorine Gas Reaction Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. Bromine is less reactive, means it. These reactions are usually spontaneous. Bromine + chlorine = bromine monochloride. Chlorine, bromine or iodine. Bromine Chlorine Gas Reaction.
From ar.inspiredpencil.com
Bromine Gas Equation Bromine Chlorine Gas Reaction Bromine is less reactive, means it. Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. These reactions are usually spontaneous. The relative lower reactivity of bromine. Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED Aqueous sodium chloride (NaCl) and liquid bromine (Brz are formed by the reaction of Bromine Chlorine Gas Reaction Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Bromine is less reactive, means it. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of. Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED According to the following reaction, how many grams of chlorine gas are necessary to Bromine Chlorine Gas Reaction Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. These reactions are usually spontaneous. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Bromine + chlorine = bromine monochloride. The relative lower reactivity of bromine makes it exhibits a. Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED According to the following reaction, how many grams of chlorine gas are required for the Bromine Chlorine Gas Reaction Chlorine, bromine or iodine can be added to an alkene. Bromine is less reactive, means it. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. The reaction between c=c double bond and bromine (br 2). Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED Sodium chloride reacts with bromine gas, mathrm{Br}_{2}, to produce sodium bromide Bromine Chlorine Gas Reaction Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Bromine is less reactive, means it. Bromine + chlorine = bromine monochloride. Chlorine, bromine or iodine can. Bromine Chlorine Gas Reaction.
From www.researchgate.net
Simplified scheme of bromine explosion and ozone destruction reactions... Download Scientific Bromine Chlorine Gas Reaction Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Different possible mechanisms for the gas phase reaction of formation of bromine chloride from. Bromine Chlorine Gas Reaction.
From klassyqzs.blob.core.windows.net
Bromine And Chlorine Mixed Together at Emily Simpson blog Bromine Chlorine Gas Reaction Bromine + chlorine = bromine monochloride. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Bromine is less reactive, means it. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Different possible mechanisms for the gas phase reaction of formation. Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED When hydrobromic acid (HBr) reacts with chlorine gas, bromine and hydrochloric acid are Bromine Chlorine Gas Reaction Bromine is less reactive, means it. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Chlorine, bromine or iodine can be added to an alkene. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Br + cl = brcl is. Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED Q1 State what would be observed, if anything, in the following reactions Chlorine gas Bromine Chlorine Gas Reaction These reactions are usually spontaneous. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Br + cl = brcl. Bromine Chlorine Gas Reaction.
From www.coursehero.com
[Solved] The reaction of bromine gas with chlorine gas, shown here, has a Kc... Course Hero Bromine Chlorine Gas Reaction Chlorine, bromine or iodine can be added to an alkene. Bromine + chlorine = bromine monochloride. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Bromine is less reactive, means it. Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine. Bromine Chlorine Gas Reaction.
From www.masterorganicchemistry.com
Bromination of Alkenes The Mechanism Master Organic Chemistry Bromine Chlorine Gas Reaction Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. These reactions are usually spontaneous. Bromine is less reactive, means it. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Different possible mechanisms. Bromine Chlorine Gas Reaction.
From socratic.org
How do you write the equation for this reaction Aluminum bromide and chlorine gas react to form Bromine Chlorine Gas Reaction When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Chlorine, bromine or iodine can be added to an alkene. Bromine is less reactive, means it. These reactions are usually spontaneous. Bromine + chlorine = bromine monochloride. The reaction between c=c double bond and bromine. Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED Chlorine and bromine react in the dark with alkenes. The reaction shown below affords a Bromine Chlorine Gas Reaction Bromine + chlorine = bromine monochloride. These reactions are usually spontaneous. Chlorine, bromine or iodine can be added to an alkene. Bromine is less reactive, means it. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Br + cl = brcl is a synthesis. Bromine Chlorine Gas Reaction.
From www.youtube.com
NaBr+Cl2=NaCl+Br2 Balanced EquationSodium Bromide+Chlorine=Sodium Chloride+Bromine YouTube Bromine Chlorine Gas Reaction Chlorine, bromine or iodine can be added to an alkene. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Bromine + chlorine = bromine monochloride. Bromine is less reactive, means it. The relative lower reactivity of bromine makes it exhibits a much greater selectivity.. Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED For the following reaction, 28.1 grams of bromine are allowed to react with 16.4 grams Bromine Chlorine Gas Reaction These reactions are usually spontaneous. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Bromine + chlorine = bromine monochloride. Br + cl = brcl is a synthesis reaction where one. Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED Chlorine gas reacts with aqueous sodium bromide to produce aqueous sodium chloride and Bromine Chlorine Gas Reaction Chlorine, bromine or iodine can be added to an alkene. Bromine + chlorine = bromine monochloride. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert. Bromine Chlorine Gas Reaction.
From www.chegg.com
Solved 9. For the reaction of sodium bromide with chlorine Bromine Chlorine Gas Reaction The relative lower reactivity of bromine makes it exhibits a much greater selectivity. These reactions are usually spontaneous. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Bromine + chlorine = bromine monochloride. When alkenes (also known as olefins) are treated with bromine (br. Bromine Chlorine Gas Reaction.
From www.alamy.com
Synthesis reaction sodium chloride formation of sodium metal and chlorine gas. Types of Bromine Chlorine Gas Reaction Bromine + chlorine = bromine monochloride. Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. These reactions. Bromine Chlorine Gas Reaction.
From www.youtube.com
Type of Reaction for NaBr + Cl2 = NaCl + Br2 YouTube Bromine Chlorine Gas Reaction Bromine is less reactive, means it. Chlorine, bromine or iodine can be added to an alkene. Bromine + chlorine = bromine monochloride. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. The relative lower reactivity of bromine makes it exhibits a much greater selectivity.. Bromine Chlorine Gas Reaction.
From www.alamy.com
Bubbling Chlorine Gas into Sodium Bromide to Yield Bromine Stock Photo Alamy Bromine Chlorine Gas Reaction Chlorine, bromine or iodine can be added to an alkene. The reaction between c=c double bond and bromine (br 2) can be used as a test for the presence of alkene in an unknown sample. Bromine + chlorine = bromine monochloride. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Different possible mechanisms for the gas. Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED For the following reaction, 6.37 grams of chlorine gas are mixed with excess bromine Bromine Chlorine Gas Reaction When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Chlorine, bromine or iodine can be added to an alkene. The relative lower reactivity. Bromine Chlorine Gas Reaction.
From www.numerade.com
SOLVED Part A Enter a balanced equation for the reaction of chlorine gas with bromine gas Bromine Chlorine Gas Reaction When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. These reactions are usually spontaneous. Bromine + chlorine = bromine monochloride. Bromine is less reactive, means it. Chlorine, bromine or iodine can be added to an alkene. Br + cl = brcl is a synthesis. Bromine Chlorine Gas Reaction.
From exodbccbq.blob.core.windows.net
Chlorine And Bromine Bond Type at Alfredo Kaminski blog Bromine Chlorine Gas Reaction Bromine is less reactive, means it. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. The relative lower reactivity of bromine makes it exhibits a much greater selectivity. Chlorine, bromine or iodine can be added to an alkene. The reaction between c=c double bond. Bromine Chlorine Gas Reaction.
From www.slideserve.com
PPT EXTRACTION OF BROMINE FROM SEA WATER PowerPoint Presentation ID318185 Bromine Chlorine Gas Reaction When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent [note 1] such as carbon. Bromine is less reactive, means it. Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. These reactions are usually spontaneous. Chlorine, bromine or. Bromine Chlorine Gas Reaction.
From solvedlib.com
9/The reaction of bromine gas with chlorine gas, show… SolvedLib Bromine Chlorine Gas Reaction Br + cl = brcl is a synthesis reaction where one mole of bromine [br] and one mole of chlorine [cl]. Chlorine, bromine or iodine can be added to an alkene. These reactions are usually spontaneous. Bromine is less reactive, means it. Bromine + chlorine = bromine monochloride. The relative lower reactivity of bromine makes it exhibits a much greater. Bromine Chlorine Gas Reaction.
From www.solutionspile.com
[Solved] 4. At a certain temperature, the equilibrium con Bromine Chlorine Gas Reaction Chlorine, bromine or iodine can be added to an alkene. Bromine is less reactive, means it. Different possible mechanisms for the gas phase reaction of formation of bromine chloride from bromine and chlorine are. Bromine + chlorine = bromine monochloride. When alkenes (also known as olefins) are treated with bromine (br 2) or chlorine (cl 2) in an inert solvent. Bromine Chlorine Gas Reaction.