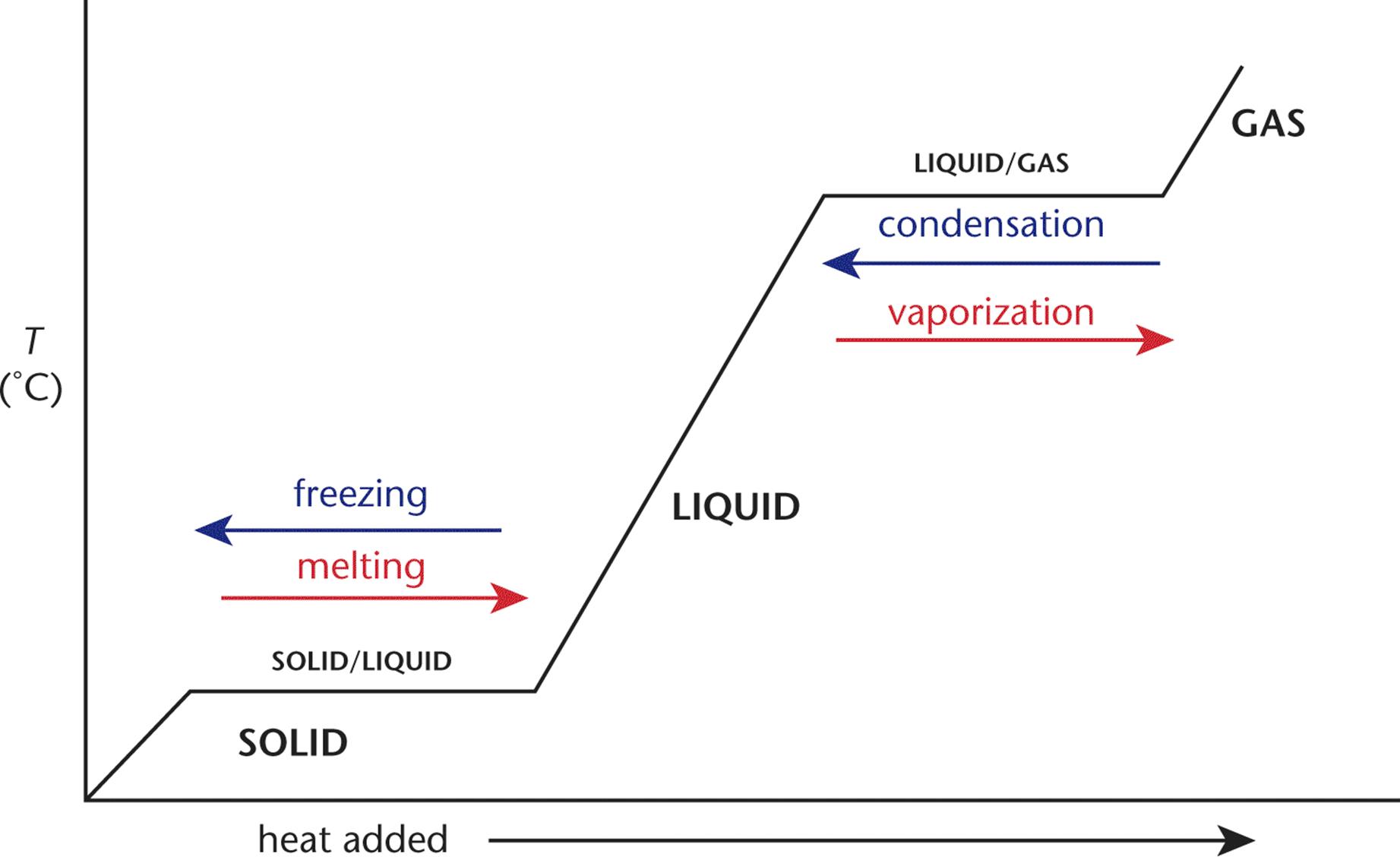
Chemistry Heating Curve Questions . Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. The heating curve shown above is a plot of temperature vs time. Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. What is the freezing temperature of the above substance? Use phase diagrams to identify stable phases at given temperatures and. What is the melting temperature of the above substance? Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the. Study with quizlet and memorize flashcards containing terms like it's in the solid state. The temperature is going up and the. It represents the heating of substance x at a constant rate of. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Explain the construction and use of a typical phase diagram.
from schoolbag.info
The heating curve shown above is a plot of temperature vs time. Study with quizlet and memorize flashcards containing terms like it's in the solid state. Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. Explain the construction and use of a typical phase diagram. Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the. What is the freezing temperature of the above substance? Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Use phase diagrams to identify stable phases at given temperatures and. What is the melting temperature of the above substance? Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram:
Figure 7.7. Heating Curve for a Single Compound
Chemistry Heating Curve Questions The heating curve shown above is a plot of temperature vs time. Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the. Study with quizlet and memorize flashcards containing terms like it's in the solid state. What is the melting temperature of the above substance? The temperature is going up and the. Explain the construction and use of a typical phase diagram. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: The heating curve shown above is a plot of temperature vs time. Use phase diagrams to identify stable phases at given temperatures and. It represents the heating of substance x at a constant rate of. What is the freezing temperature of the above substance?
From www.numerade.com
The following graph is a heating curve for chloroform, a solvent for fats, oils, and waxes a Chemistry Heating Curve Questions The heating curve shown above is a plot of temperature vs time. What is the melting temperature of the above substance? Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of. Chemistry Heating Curve Questions.
From answerlibraryweizzz.z13.web.core.windows.net
Chemistry Heating Curve Worksheet Answers Chemistry Heating Curve Questions Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. The heating curve shown above is a plot of temperature vs time. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: It represents the heating of substance. Chemistry Heating Curve Questions.
From worksheets.clipart-library.com
Heating Curves Worksheet Freezing, Boiling, and Melting Points Worksheets Library Chemistry Heating Curve Questions Explain the construction and use of a typical phase diagram. The temperature is going up and the. What is the freezing temperature of the above substance? It represents the heating of substance x at a constant rate of. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Heating&cooling curves. Chemistry Heating Curve Questions.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Chemistry Heating Curve Questions Explain the construction and use of a typical phase diagram. The heating curve shown above is a plot of temperature vs time. The temperature is going up and the. What is the melting temperature of the above substance? Use phase diagrams to identify stable phases at given temperatures and. Elemental carbon has one gas phase, one liquid phase, and three. Chemistry Heating Curve Questions.
From quizizz.com
Heating Curves questions & answers for quizzes and tests Quizizz Chemistry Heating Curve Questions Study with quizlet and memorize flashcards containing terms like it's in the solid state. What is the melting temperature of the above substance? What is the freezing temperature of the above substance? Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the. Elemental carbon has one gas phase, one liquid phase,. Chemistry Heating Curve Questions.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV ] Chemistry YouTube Chemistry Heating Curve Questions What is the freezing temperature of the above substance? Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Study with quizlet and memorize flashcards containing terms like it's in the. Chemistry Heating Curve Questions.
From spmchemistry.blog.onlinetuition.com.my
Cooling Curve SPM Chemistry Chemistry Heating Curve Questions What is the freezing temperature of the above substance? The temperature is going up and the. It represents the heating of substance x at a constant rate of. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. The heating curve shown above is a plot of temperature. Chemistry Heating Curve Questions.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Chemistry Heating Curve Questions Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. The temperature is going up and the. It represents the heating of substance x at a constant rate of. Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the.. Chemistry Heating Curve Questions.
From schematicdiagramglocer.z19.web.core.windows.net
Heating Curve Chemistry Diagram Chemistry Heating Curve Questions Explain the construction and use of a typical phase diagram. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Use phase diagrams to identify stable phases at given temperatures and. It represents the heating of substance x at a constant rate of. What is the melting temperature of the. Chemistry Heating Curve Questions.
From spmchemistry.blog.onlinetuition.com.my
Three States of Matter Structured Question 4 SPM Chemistry Chemistry Heating Curve Questions Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. What is the melting temperature of the above substance? What is the freezing temperature of the above substance? Use phase diagrams to identify stable phases at given temperatures and. It represents the heating of substance x at a. Chemistry Heating Curve Questions.
From quizizz.com
Heating and Cooling Curves Chemistry Quiz Quizizz Chemistry Heating Curve Questions What is the melting temperature of the above substance? Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: The temperature is going up and the. Explain the construction and use of a typical phase diagram. Plots of the temperature of a substance versus heat added or versus heating time. Chemistry Heating Curve Questions.
From www.pinterest.com
Heating curve calculation (benzene) Worksheets, Printable preschool worksheets, Chemistry Chemistry Heating Curve Questions What is the freezing temperature of the above substance? It represents the heating of substance x at a constant rate of. Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. Study with quizlet and memorize flashcards containing terms like it's in the solid state. What is the. Chemistry Heating Curve Questions.
From studylib.net
A.2 Heat Curves Phase diagram Worksheet Key Chemistry Heating Curve Questions Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Use phase diagrams to identify stable phases at given temperatures and. What is the freezing temperature of the above substance? The heating curve shown above is a plot of temperature vs time. Study with quizlet and memorize flashcards. Chemistry Heating Curve Questions.
From quizizz.com
Heating Curve Graphs Chemistry Quizizz Chemistry Heating Curve Questions Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. The temperature is going up and the. What is the freezing temperature of the above substance? Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called.. Chemistry Heating Curve Questions.
From www.albert.io
Heating Curve and Specific Heat Chemistry Practice Albert Chemistry Heating Curve Questions The heating curve shown above is a plot of temperature vs time. Study with quizlet and memorize flashcards containing terms like it's in the solid state. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Assume that the heat necessary to evaporate isopentane is only from the. Chemistry Heating Curve Questions.
From schoolbag.info
Figure 7.7. Heating Curve for a Single Compound Chemistry Heating Curve Questions Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the. Use phase diagrams to identify stable phases at given temperatures and. What is the melting temperature of the above. Chemistry Heating Curve Questions.
From lessonlibsertularia.z22.web.core.windows.net
Heating And Cooling Curves Explained Chemistry Heating Curve Questions The temperature is going up and the. It represents the heating of substance x at a constant rate of. The heating curve shown above is a plot of temperature vs time. Explain the construction and use of a typical phase diagram. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of. Chemistry Heating Curve Questions.
From www.youtube.com
heating and cooling curves worksheet video 1 YouTube Chemistry Heating Curve Questions Explain the construction and use of a typical phase diagram. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Study with quizlet and memorize flashcards containing terms like it's in the solid state. Elemental carbon has one gas phase, one liquid phase, and three different solid phases,. Chemistry Heating Curve Questions.
From www.studocu.com
82218 heating and cooling curve answers General Chemistry Lecture Studocu Chemistry Heating Curve Questions Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the. It represents the heating of substance x at a constant rate of. Explain the construction and use of a typical phase diagram. Use phase diagrams to identify stable phases at given temperatures and. Plots of the temperature of a substance versus. Chemistry Heating Curve Questions.
From db-excel.com
Chemistry Heating Curve Worksheet — Chemistry Heating Curve Questions Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. The heating curve shown above is a plot of temperature vs time. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Explain the construction and use of. Chemistry Heating Curve Questions.
From www.chegg.com
Solved CHEMISTRY HEATING CURVE WORKSHEET Heating Curve of Chemistry Heating Curve Questions Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Use phase diagrams to identify stable phases at given temperatures and. It represents the heating of substance x at a constant rate of. Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a. Chemistry Heating Curve Questions.
From www.coursehero.com
[Solved] Worksheet 9.3 HEATING CURVES 1. a) What... Course Hero Chemistry Heating Curve Questions The temperature is going up and the. What is the melting temperature of the above substance? Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: The heating. Chemistry Heating Curve Questions.
From printablelistquinta.z21.web.core.windows.net
Heating And Cooling Curve Questions Chemistry Heating Curve Questions Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. Use phase diagrams to identify stable phases at given temperatures and. Heating&cooling curves a)ab b)bc c)de. Chemistry Heating Curve Questions.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Chemistry Heating Curve Questions What is the freezing temperature of the above substance? Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the. Plots of the temperature of a substance versus heat added or versus. Chemistry Heating Curve Questions.
From www.chegg.com
Solved CHEMISTRY HEATING CURVE WORKSHEET Heating Curve of Chemistry Heating Curve Questions Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: The heating curve shown above is a plot of temperature vs time. What is the freezing temperature of. Chemistry Heating Curve Questions.
From www.smartexamresources.com
IGCSE Chemistry Notes Solids, Liquids And Gases Smart Exam Resources Chemistry Heating Curve Questions Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Use phase diagrams to identify stable phases at given temperatures and. Explain the construction and use. Chemistry Heating Curve Questions.
From heatinggondon.blogspot.com
Heating Heating Curve Worksheet Chemistry Heating Curve Questions The heating curve shown above is a plot of temperature vs time. Use phase diagrams to identify stable phases at given temperatures and. What is the freezing temperature of the above substance? Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Elemental carbon has one gas phase,. Chemistry Heating Curve Questions.
From printablelibmolines.z13.web.core.windows.net
Heating Curve Of Water Worksheet Chemistry Heating Curve Questions Study with quizlet and memorize flashcards containing terms like it's in the solid state. The temperature is going up and the. Explain the construction and use of a typical phase diagram. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Plots of the temperature of a substance versus heat. Chemistry Heating Curve Questions.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Chemistry Heating Curve Questions The temperature is going up and the. What is the melting temperature of the above substance? Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Use phase diagrams to identify stable phases at given temperatures and. Study with quizlet and memorize flashcards containing terms like it's in. Chemistry Heating Curve Questions.
From www.chegg.com
Solved CHEMISTRY HEATING CURVE WORKSHEET Heating Curve of Chemistry Heating Curve Questions Study with quizlet and memorize flashcards containing terms like it's in the solid state. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: What is the melting temperature of the above substance? What is the freezing temperature of the above substance? It represents the heating of substance x at. Chemistry Heating Curve Questions.
From studylib.net
Heating Curve Worksheet Chemistry Heating Curve Questions The heating curve shown above is a plot of temperature vs time. Plots of the temperature of a substance versus heat added or versus heating time at a constant rate of heating are called. Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the. What is the melting temperature of the. Chemistry Heating Curve Questions.
From studylib.net
Dougherty Valley HS AP Chemistry Name Heating Curve Practice Chemistry Heating Curve Questions Use phase diagrams to identify stable phases at given temperatures and. Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c (δh. What is the melting temperature of the above substance? The temperature is going up and the. Study with quizlet and memorize flashcards containing terms like it's in. Chemistry Heating Curve Questions.
From www.pinterest.com
heating curve questions Thermodynamics, This or that questions, Intermolecular force Chemistry Heating Curve Questions What is the melting temperature of the above substance? Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the. Use phase diagrams to identify stable phases at given temperatures and. Assume that the heat necessary to evaporate isopentane is only from the block and that isopentane already vaporizes at 25.0 °c. Chemistry Heating Curve Questions.
From www.chegg.com
Solved Q5 Heating Curve 2 Points Consider the following Chemistry Heating Curve Questions What is the melting temperature of the above substance? Heating&cooling curves a)ab b)bc c)de d)ef 31.the graph below represents the uniform heating of a substance, starting with the. Use phase diagrams to identify stable phases at given temperatures and. Study with quizlet and memorize flashcards containing terms like it's in the solid state. Plots of the temperature of a substance. Chemistry Heating Curve Questions.
From exobuhknu.blob.core.windows.net
Heating Curve Of Substance X at Nina Edwards blog Chemistry Heating Curve Questions It represents the heating of substance x at a constant rate of. Use phase diagrams to identify stable phases at given temperatures and. Elemental carbon has one gas phase, one liquid phase, and three different solid phases, as shown in the phase diagram: Plots of the temperature of a substance versus heat added or versus heating time at a constant. Chemistry Heating Curve Questions.