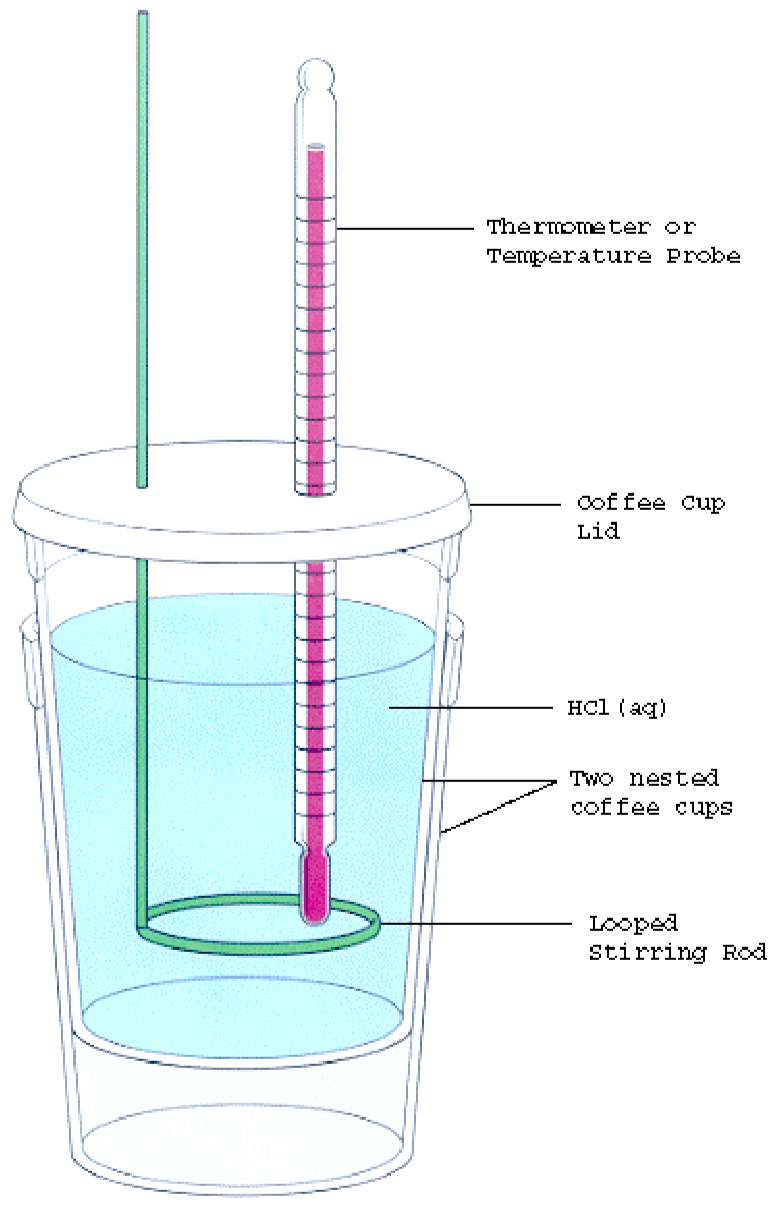
Calorimetry In Chemistry . Learn about the different types of. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Learn about the three methods for measuring heat in chemical reactions: You will monitor the change in heat. Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature change, and specific heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Chemistry document from university of notre dame, 3 pages, calorimetry purpose: Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Temperature change, heat conduction, and. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn about different types of calorimeters, the calorimetry. Find out the necessary parts of a. For example, when an exothermic. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is a technique to measure the heat involved in chemical or physical processes.
from chem.libretexts.org
Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature change, and specific heat. Learn about the different types of. Learn about the three methods for measuring heat in chemical reactions: Find out the necessary parts of a. Chemistry document from university of notre dame, 3 pages, calorimetry purpose: Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about different types of calorimeters, the calorimetry. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances.
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts
Calorimetry In Chemistry Find out the necessary parts of a. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Find out the necessary parts of a. You will monitor the change in heat. Learn about the three methods for measuring heat in chemical reactions: Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature change, and specific heat. Learn about the different types of. For example, when an exothermic. Chemistry document from university of notre dame, 3 pages, calorimetry purpose: Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is a technique to measure the heat involved in chemical or physical processes. Temperature change, heat conduction, and. Learn about different types of calorimeters, the calorimetry.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry In Chemistry Calorimetry is a technique to measure the heat involved in chemical or physical processes. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Find out the necessary parts of a. Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature change, and. Calorimetry In Chemistry.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry In Chemistry Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Calorimetry is a technique to measure the heat involved in chemical or physical processes. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. You will monitor the change in heat. For. Calorimetry In Chemistry.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Chemistry Calorimetry In Chemistry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Find out the necessary parts of a. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Chemistry document from university of notre dame, 3 pages, calorimetry purpose: Temperature change, heat conduction, and.. Calorimetry In Chemistry.
From www.learner.org
The Energy in Chemical Reactions Thermodynamics and Enthalpy Annenberg Learner Calorimetry In Chemistry Find out the necessary parts of a. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. For example, when an exothermic. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about the different types of. Learn how to use calorimetry. Calorimetry In Chemistry.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimetry In Chemistry Learn about the different types of. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Chemistry document from university of notre dame, 3 pages, calorimetry purpose: Find out the necessary parts of a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn. Calorimetry In Chemistry.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry In Chemistry Chemistry document from university of notre dame, 3 pages, calorimetry purpose: Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is an application of the first law of thermodynamics to measure. Calorimetry In Chemistry.
From www.hanlin.com
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry In Chemistry Calorimetry is a technique to measure the heat involved in chemical or physical processes. Learn about the different types of. Learn about the three methods for measuring heat in chemical reactions: Learn about different types of calorimeters, the calorimetry. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. Calorimetry In Chemistry.
From www.animalia-life.club
Calorimeter Diagram Calorimetry In Chemistry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic. Calorimetry is a technique to measure the heat involved in chemical or physical processes. Temperature change, heat conduction, and. A calorimeter is a device used to measure the amount of heat involved in a chemical or. Calorimetry In Chemistry.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimetry In Chemistry Calorimetry is a technique to measure the heat involved in chemical or physical processes. You will monitor the change in heat. Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature change, and specific heat. Learn how to measure the amount of heat involved in a chemical or physical process using a. Calorimetry In Chemistry.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Calorimetry In Chemistry Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. For example, when an exothermic. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Temperature change, heat conduction, and. Learn about the different types of. Calorimetry is a technique to measure. Calorimetry In Chemistry.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry In Chemistry Learn about the three methods for measuring heat in chemical reactions: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Learn about different types of calorimeters, the calorimetry. Learn how to use. Calorimetry In Chemistry.
From www.youtube.com
Calorimetry (AQA A level Chemistry) YouTube Calorimetry In Chemistry Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. You will monitor the change in heat. Calorimetry is a technique to measure the heat involved in chemical or physical. Calorimetry In Chemistry.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry In Chemistry Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature change, and specific heat. Temperature change, heat conduction, and. A calorimeter is a device used to measure the amount of. Calorimetry In Chemistry.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID1084959 Calorimetry In Chemistry Learn about different types of calorimeters, the calorimetry. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature change, and specific heat. Temperature change, heat conduction, and. Learn. Calorimetry In Chemistry.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry In Chemistry Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature change, and specific heat. Temperature change, heat conduction, and. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about the different types of. Chemistry document from university of notre dame, 3. Calorimetry In Chemistry.
From ar.inspiredpencil.com
Calorimeter Diagram Calorimetry In Chemistry Temperature change, heat conduction, and. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Learn about the different types of. Learn about different types of calorimeters, the calorimetry. Find out the necessary parts of a. Chemistry document from university of notre dame, 3 pages, calorimetry. Calorimetry In Chemistry.
From themasterchemistry.com
Calorimeter Types, Principle, Working, Uses Calorimetry In Chemistry Temperature change, heat conduction, and. Chemistry document from university of notre dame, 3 pages, calorimetry purpose: Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Find out the necessary. Calorimetry In Chemistry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry In Chemistry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature change, and specific heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimetry In Chemistry.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimetry In Chemistry Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. You will monitor the change in heat. Learn about the three methods for measuring heat in chemical reactions: Calorimetry is a technique to measure the heat involved in chemical or physical processes. Calorimetry is a field of thermochemistry that measures the amount. Calorimetry In Chemistry.
From www.youtube.com
050 Calorimetry YouTube Calorimetry In Chemistry Chemistry document from university of notre dame, 3 pages, calorimetry purpose: Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Learn about different types of calorimeters, the calorimetry. Learn about the different types of. Learn about the three methods for measuring heat in chemical reactions: Calorimetry is a technique to measure. Calorimetry In Chemistry.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry In Chemistry Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature change, and specific heat. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. Chemistry document from university of notre dame, 3 pages, calorimetry purpose: Temperature change, heat conduction, and. Calorimetry is a technique. Calorimetry In Chemistry.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry In Chemistry Learn about the three methods for measuring heat in chemical reactions: Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. You will monitor the change in heat. Find out the necessary parts of a. Chemistry. Calorimetry In Chemistry.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry In Chemistry For example, when an exothermic. Learn about the different types of. Chemistry document from university of notre dame, 3 pages, calorimetry purpose: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat. Calorimetry In Chemistry.
From sites.google.com
Calorimetry Preliminary HSC Chemistry Calorimetry In Chemistry Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn about the different types of. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. For example, when an exothermic. Learn about different types of calorimeters, the calorimetry. Calorimetry is an application of the. Calorimetry In Chemistry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry In Chemistry Find out the necessary parts of a. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn about the different types of. Learn how to use ideal and real calorimeters, coffee cup calorimetry, and. Calorimetry In Chemistry.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Calorimetry In Chemistry Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature change, and specific heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical. Calorimetry In Chemistry.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry In Chemistry You will monitor the change in heat. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to calculate heat, temperature. Calorimetry In Chemistry.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry In Chemistry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Learn about the different types of. Learn about the three methods for measuring heat in chemical reactions: Learn about different types of calorimeters, the calorimetry. Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters.. Calorimetry In Chemistry.
From www.scribd.com
calorimetry Chemical Engineering Chemical Process Engineering Calorimetry In Chemistry You will monitor the change in heat. Learn about the three methods for measuring heat in chemical reactions: Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Calorimetry is a technique to measure the heat involved in chemical or physical processes. Learn about different types of calorimeters, the calorimetry. For. Calorimetry In Chemistry.
From users.highland.edu
Calorimetry Calorimetry In Chemistry Find out the necessary parts of a. Learn about the three methods for measuring heat in chemical reactions: For example, when an exothermic. Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. You will monitor the change in heat. Learn about the different types of. Calorimetry is a field of thermochemistry. Calorimetry In Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry Calorimetry In Chemistry Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. You will monitor the change in heat. Temperature change, heat conduction, and. For example, when an exothermic. Learn about the three methods for measuring heat in chemical reactions: Find out the necessary parts of a. Learn how to. Calorimetry In Chemistry.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2692866 Calorimetry In Chemistry Learn how to measure the amount of heat involved in a chemical or physical process using a calorimeter. For example, when an exothermic. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Learn how to use ideal and real calorimeters, coffee cup calorimetry, and bomb calorimetry to. Calorimetry In Chemistry.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation & Application Calorimetry In Chemistry Temperature change, heat conduction, and. Calorimetry is a technique to measure the heat involved in chemical or physical processes. For example, when an exothermic. Learn about the three methods for measuring heat in chemical reactions: Learn how to use calorimetry to measure enthalpy changes in chemical processes using devices called calorimeters. Learn how to use ideal and real calorimeters, coffee. Calorimetry In Chemistry.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry In Chemistry Learn about the different types of. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of substances. Chemistry document from university of notre dame, 3 pages, calorimetry purpose: A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how. Calorimetry In Chemistry.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.3 Calorimetry翰林国际教育 Calorimetry In Chemistry Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical reaction. Find out the necessary parts of a. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn about different types of calorimeters, the calorimetry. Chemistry document from university of notre dame,. Calorimetry In Chemistry.