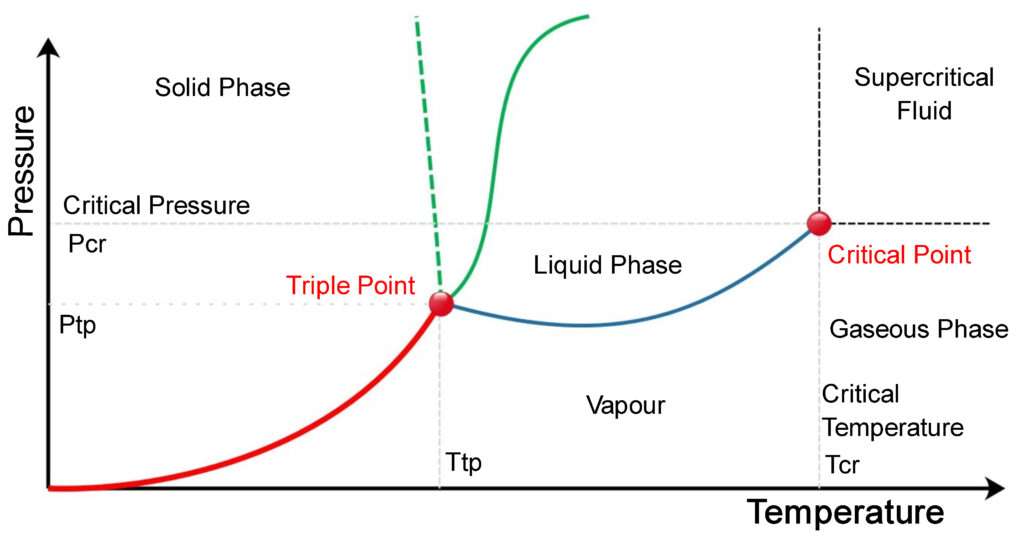
Characteristics Of Triple Point . The triple point is the temperature and pressure at which three states of matter exist in equilibrium. The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. Updated on november 18, 2019. The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and solid) coexist in. Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. The critical point is also a combination of temperature and pressure, but. The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. At the triple point, all three phases (solid, liquid, gas). 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The triple point is used as a fixed reference point to define the kelvin temperature scale.
from chemicalengineeringworld.com
The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium. The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. The triple point is the temperature and pressure at which three states of matter exist in equilibrium. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The critical point is also a combination of temperature and pressure, but. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and solid) coexist in. At the triple point, all three phases (solid, liquid, gas). Updated on november 18, 2019. The triple point is used as a fixed reference point to define the kelvin temperature scale.
Critical Point and Triple Point Chemical Engineering World
Characteristics Of Triple Point The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and solid) coexist in. The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium. The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. At the triple point, all three phases (solid, liquid, gas). Updated on november 18, 2019. The critical point is also a combination of temperature and pressure, but. The triple point is used as a fixed reference point to define the kelvin temperature scale. The triple point is the temperature and pressure at which three states of matter exist in equilibrium.
From www.youtube.com
Critical Point Triple Point Vs. Critical Point YouTube Characteristics Of Triple Point 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid,. Characteristics Of Triple Point.
From www.researchgate.net
The measurementrelated standard uncertainty for the xenon triple point Characteristics Of Triple Point Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. The triple point of a substance is the specific condition at which all three phases (solid,. Characteristics Of Triple Point.
From www.doubtnut.com
In the phase diagram showns, the point Q corresponds to the triple Characteristics Of Triple Point The triple point is the temperature and pressure at which three states of matter exist in equilibrium. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas). Characteristics Of Triple Point.
From www.thoughtco.com
Triple Point Definition and Example (Chemistry) Characteristics Of Triple Point 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and solid) coexist in. The triple point is a thermodynamic term that refers to a specific combination of. Characteristics Of Triple Point.
From chemicalengineeringworld.com
Critical Point and Triple Point Chemical Engineering World Characteristics Of Triple Point At the triple point, all three phases (solid, liquid, gas). The triple point is used as a fixed reference point to define the kelvin temperature scale. Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. Updated on november 18, 2019. In chemistry and physics, the. Characteristics Of Triple Point.
From www.vrogue.co
Phase Diagram Of Water Triple Point vrogue.co Characteristics Of Triple Point The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium. Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. Updated on november 18, 2019. In chemistry and physics, the triple point is. Characteristics Of Triple Point.
From sciencenotes.org
Triple Point Definition Triple Point of Water Characteristics Of Triple Point The triple point is used as a fixed reference point to define the kelvin temperature scale. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas). Characteristics Of Triple Point.
From favpng.com
Triple Point Phase Diagram Phase Transition, PNG, 600x600px, Triple Characteristics Of Triple Point Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The critical point is also a combination of temperature and pressure, but.. Characteristics Of Triple Point.
From www.youtube.com
Determining Triple Point YouTube Characteristics Of Triple Point The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and solid) coexist in. The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure. Characteristics Of Triple Point.
From www.slideserve.com
PPT Physical Transformations of Pure Substances PowerPoint Characteristics Of Triple Point The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium. The critical point is also a combination of temperature and pressure, but. 43 rows in thermodynamics, the. Characteristics Of Triple Point.
From www.youtube.com
Triple Point in a Phase Diagram // Thermodynamics Class 47 YouTube Characteristics Of Triple Point Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and solid) coexist in. Updated on november 18, 2019. In chemistry and physics, the triple point is the. Characteristics Of Triple Point.
From www.slideserve.com
PPT Triple Point Plot PowerPoint Presentation, free download ID6071904 Characteristics Of Triple Point Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. The critical point is also a combination of temperature and pressure, but. The triple point of. Characteristics Of Triple Point.
From www.animalia-life.club
Triple Point Chemistry Characteristics Of Triple Point Updated on november 18, 2019. The critical point is also a combination of temperature and pressure, but. The triple point is the temperature and pressure at which three states of matter exist in equilibrium. The triple point is used as a fixed reference point to define the kelvin temperature scale. Far more reproducible than the melting point of ice, which. Characteristics Of Triple Point.
From www.youtube.com
Triple point YouTube Characteristics Of Triple Point The triple point is used as a fixed reference point to define the kelvin temperature scale. The triple point is the temperature and pressure at which three states of matter exist in equilibrium. The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium. 43 rows in thermodynamics,. Characteristics Of Triple Point.
From www.youtube.com
Triple Point of Water Triple Point Diagram Freeze Drying Process Characteristics Of Triple Point 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium. The triple point is used as a fixed reference point to define the. Characteristics Of Triple Point.
From quizlet.com
The triple point of hydrogen occurs at a temperature of 13.8 Quizlet Characteristics Of Triple Point The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium. The critical point is also a combination of temperature and pressure, but. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in.. Characteristics Of Triple Point.
From www.slideserve.com
PPT Triple Point Plot PowerPoint Presentation, free download ID6071904 Characteristics Of Triple Point The critical point is also a combination of temperature and pressure, but. The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and solid) coexist in. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. 43. Characteristics Of Triple Point.
From byjus.com
What is triple point?Explain in detail Characteristics Of Triple Point Updated on november 18, 2019. At the triple point, all three phases (solid, liquid, gas). The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and solid) coexist in. The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium.. Characteristics Of Triple Point.
From www.researchgate.net
Triple Point Discovery Wheel Download Scientific Diagram Characteristics Of Triple Point In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. The critical point is also a combination of temperature and pressure, but. 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and. Characteristics Of Triple Point.
From www.youtube.com
CONCEPT OF TRIPLE POINT TRIPLE POINT OF WATER 💧 YouTube Characteristics Of Triple Point In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and solid) coexist in. 43 rows in thermodynamics, the triple point of a substance is the temperature. Characteristics Of Triple Point.
From gamesmartz.com
Triple Point Definition & Image GameSmartz Characteristics Of Triple Point The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium. At the triple point, all three phases (solid, liquid, gas). In chemistry and physics, the triple point. Characteristics Of Triple Point.
From stock.adobe.com
Triple phase diagram of water Stock Vector Adobe Stock Characteristics Of Triple Point Updated on november 18, 2019. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. The triple point is used as. Characteristics Of Triple Point.
From klasmeier.engineer
Water Triple Point Thomas Klasmeier Characteristics Of Triple Point Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and solid) coexist in. 43 rows in thermodynamics, the triple point of a substance is the temperature and. Characteristics Of Triple Point.
From esm.rkriz.net
Triple point thermodynamic processes associated with Gibbs free energy Characteristics Of Triple Point 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). At the triple point, all three phases (solid, liquid, gas). The triple point is used as a fixed reference point to define the kelvin temperature scale. The triple point of a substance is the specific condition. Characteristics Of Triple Point.
From byjus.com
What is triple point?Explain in detail Characteristics Of Triple Point In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. The critical point is also a combination of temperature and pressure, but. The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. The triple point. Characteristics Of Triple Point.
From www.hpacmag.com
Fig 3 Triple Point_opt HPAC MagazineHPAC Magazine Characteristics Of Triple Point At the triple point, all three phases (solid, liquid, gas). 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). The critical point is also a combination of temperature and pressure, but. The triple point is the temperature and pressure at which three states of matter. Characteristics Of Triple Point.
From brainly.in
What is the difference between a critical point and a triple point Characteristics Of Triple Point The critical point is also a combination of temperature and pressure, but. Updated on november 18, 2019. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. The triple point is used as a fixed reference point to define the kelvin temperature scale. Far more. Characteristics Of Triple Point.
From vaigyaniklok.home.blog
THERMODYNAMICS TRIPLE POINT VAIGYANIK LOK Characteristics Of Triple Point At the triple point, all three phases (solid, liquid, gas). The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. The critical point is also a combination of temperature and pressure, but. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor. Characteristics Of Triple Point.
From naeye.net
Triple Point of Water The Temperature Where All Three Phases Coexist Characteristics Of Triple Point Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. The triple point is used as a fixed reference point to define the kelvin temperature scale. At the triple point, all three phases (solid, liquid, gas). The triple point is the temperature and pressure at which. Characteristics Of Triple Point.
From www.researchgate.net
Typical triplepoint structure. Download Scientific Diagram Characteristics Of Triple Point The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. The triple point is the temperature and pressure at which three states of matter exist in equilibrium. The critical point is also a combination of temperature and pressure, but. Updated on november 18, 2019. Far more reproducible than the melting. Characteristics Of Triple Point.
From study.com
Phase Diagrams Critical Point, Triple Point and Phase Equilibrium Characteristics Of Triple Point In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. The triple point is used as a fixed reference point to define the kelvin temperature scale. The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and. Characteristics Of Triple Point.
From www.youtube.com
Triple point of water YouTube Characteristics Of Triple Point Far more reproducible than the melting point of ice, which depends on the amount of dissolved air and the atmospheric pressure, the triple point. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. At the triple point, all three phases (solid, liquid, gas). The. Characteristics Of Triple Point.
From www.reddit.com
ELI5 Water triple point? r/explainlikeimfive Characteristics Of Triple Point The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. In chemistry and physics, the triple point is the temperature and pressure at which solid, liquid, and vapor phases of a particular substance coexist in. Updated on november 18, 2019. The critical point is also a combination of temperature and. Characteristics Of Triple Point.
From pediaa.com
Difference Between Critical Point and Triple Point Definitions Characteristics Of Triple Point The triple point is a thermodynamic term that refers to a specific combination of temperature and pressure in which three. At the triple point, all three phases (solid, liquid, gas). 43 rows in thermodynamics, the triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid). In chemistry and physics, the triple. Characteristics Of Triple Point.
From www.youtube.com
Triple Point Phase Diagram of water YouTube Characteristics Of Triple Point The triple point of a substance is the specific temperature and pressure at which three phases (gas, liquid, and solid) coexist in. At the triple point, all three phases (solid, liquid, gas). The triple point of a substance is the specific condition at which all three phases (solid, liquid, and gas) coexist in thermodynamic equilibrium. The triple point is a. Characteristics Of Triple Point.