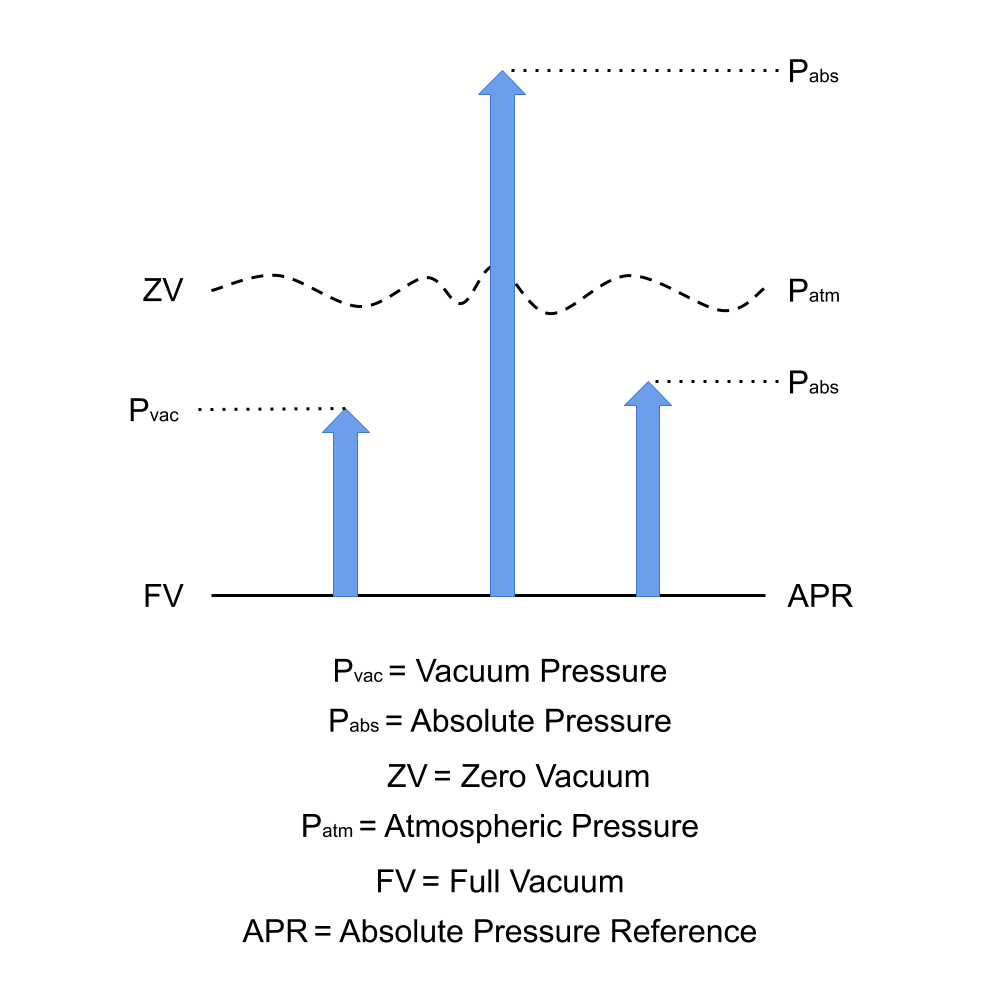
What Is Absolute Vapour Pressure . A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapor pressures are dependent only on temperature and nothing else. Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at a given. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid (or. The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor.
from howtodiscuss.com
A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at a given. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Vapor pressures are dependent only on temperature and nothing else. That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid (or. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor.
Vacuum Pressure How To Discuss
What Is Absolute Vapour Pressure Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapor pressures are dependent only on temperature and nothing else. The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid (or. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at a given. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container.
From saylordotorg.github.io
Vapor Pressure What Is Absolute Vapour Pressure A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of. What Is Absolute Vapour Pressure.
From www.researchgate.net
Vapour pressure curve (solid line), critical point (black circle) and... Download Scientific What Is Absolute Vapour Pressure That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid (or. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. The vapor. What Is Absolute Vapour Pressure.
From www.engineeringtoolbox.com
Water Thermodynamic Properties What Is Absolute Vapour Pressure Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT PUMPING 101 TASK 3 PowerPoint Presentation, free download ID5721560 What Is Absolute Vapour Pressure A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. The vapor pressure of a liquid does not depend on the amount on the liquid. What Is Absolute Vapour Pressure.
From www.conservationphysics.org
Conservation physics Fundamental microclimate concepts What Is Absolute Vapour Pressure Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at a given. Vapor pressures are dependent only on temperature and nothing else. That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid (or. A liquid’s vapour pressure is a. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT CE 374K Hydrology, Lecture 4 Atmosphere and Atmospheric water PowerPoint Presentation ID What Is Absolute Vapour Pressure A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. Vapor pressures are dependent only on temperature and nothing else. Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases. What Is Absolute Vapour Pressure.
From www.researchgate.net
Pressure ramp regimes. Absolute vapour pressure profiles demonstrating... Download Scientific What Is Absolute Vapour Pressure The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure. What Is Absolute Vapour Pressure.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Absolute Vapour Pressure Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at a given. Vapor pressures are dependent only on temperature and nothing else. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapour pressure is the pressure exerted by the. What Is Absolute Vapour Pressure.
From www.youtube.com
What is Atmospheric pressure, Gauge pressure, Absolute Pressure, Vacuum pressure & their What Is Absolute Vapour Pressure Vapor pressures are dependent only on temperature and nothing else. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); The vapor pressure of a liquid does not depend on the amount on the liquid in the container,.. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 What Is Absolute Vapour Pressure Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at a given. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid);. What Is Absolute Vapour Pressure.
From webmis.highland.cc.il.us
Vapor Pressure What Is Absolute Vapour Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Vapor pressures are dependent only on temperature and nothing else. That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid (or. Vapor pressure is. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT Lecture 11 Atmospheric moisture (Ch 5) PowerPoint Presentation, free download ID5767198 What Is Absolute Vapour Pressure Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid (or. Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at. What Is Absolute Vapour Pressure.
From www.youtube.com
CHEMISTRY 201 Using the ClausiusClapeyron equation to solve for vapor pressure YouTube What Is Absolute Vapour Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. Vapor pressure is the pressure. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT CE 374K Hydrology, Lecture 4 Atmosphere and Atmospheric water PowerPoint Presentation ID What Is Absolute Vapour Pressure Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Vapor pressure is the pressure exerted by a vapor that is. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT Dalton’s Law of Partial Pressure PowerPoint Presentation, free download ID1755772 What Is Absolute Vapour Pressure A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapor pressures are dependent only on temperature and nothing else. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. Vapor pressure is the pressure exerted by a vapor that is in. What Is Absolute Vapour Pressure.
From ecoursesonline.iasri.res.in
FM LESSON 3. PROPERTIES OF FLUID What Is Absolute Vapour Pressure The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. That is, the vapour. What Is Absolute Vapour Pressure.
From www.youtube.com
Introductory Fluid Mechanics L4 p4 Absolute, Gage, Vacuum Pressure YouTube What Is Absolute Vapour Pressure Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid (or. Vapor pressures are dependent only on temperature and. What Is Absolute Vapour Pressure.
From www.youtube.com
What is vapor pressure what is the vapor pressure of liquids Factors affecting vapor What Is Absolute Vapour Pressure The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. Vapor pressures are dependent only on temperature and nothing else. That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid (or. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it. What Is Absolute Vapour Pressure.
From www.chegg.com
Solved Definitions Partial pressure of the water vapor What Is Absolute Vapour Pressure Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at a given. Vapor pressures are dependent only on temperature and nothing else. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the. What Is Absolute Vapour Pressure.
From derangedphysiology.com
Physics of humidity and evaporation Deranged Physiology What Is Absolute Vapour Pressure That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid (or. Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at a given. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it. What Is Absolute Vapour Pressure.
From www.researchgate.net
The effect of temperature on saturation vapor pressure, actual vapor... Download Scientific What Is Absolute Vapour Pressure Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapor pressures are dependent only on temperature and nothing else. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. A. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation ID1757765 What Is Absolute Vapour Pressure Vapor pressures are dependent only on temperature and nothing else. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the. What Is Absolute Vapour Pressure.
From www.researchgate.net
Relationships between the gas temperature, absolute humidity and water... Download Table What Is Absolute Vapour Pressure Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor.. What Is Absolute Vapour Pressure.
From www.brainkart.com
Vapour pressure of liquid Solutions Chemistry What Is Absolute Vapour Pressure Vapor pressures are dependent only on temperature and nothing else. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. That. What Is Absolute Vapour Pressure.
From byjus.com
What is difference between pure vapour pressure and partial pressure What Is Absolute Vapour Pressure The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. Vapor pressures are dependent only on temperature and nothing else. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT Moisture Relationships PowerPoint Presentation, free download ID6646880 What Is Absolute Vapour Pressure The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapour pressure is the. What Is Absolute Vapour Pressure.
From www.dailymotion.com
Atmospheric Pressure, Gauge Pressure, Absolute Pressure and Vapour Pressure Shubham Kola What Is Absolute Vapour Pressure Vapor pressures are dependent only on temperature and nothing else. The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid (or. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapour pressure. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT Chapter 7 Aerosols, Sprays and Inhalations PowerPoint Presentation ID9195805 What Is Absolute Vapour Pressure Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at a given.. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT Atmospheric Moisture Relative Humidity and Dew Point PowerPoint Presentation ID9347606 What Is Absolute Vapour Pressure The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapor pressures are dependent only on temperature and nothing else. Vapor pressure is the pressure exerted by a vapor that is in. What Is Absolute Vapour Pressure.
From howtodiscuss.com
Vacuum Pressure How To Discuss What Is Absolute Vapour Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. A liquid’s vapour pressure is. What Is Absolute Vapour Pressure.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube What Is Absolute Vapour Pressure Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at a given. The vapor pressure of a liquid does not depend on the amount on the liquid in the container,. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic. What Is Absolute Vapour Pressure.
From hvacrschool.com
vapor pressure Archives HVAC School What Is Absolute Vapour Pressure Vapor pressure is the pressure exerted by a vapor that is in thermodynamic equilibrium with its condensed phases (solid or liquid) in a closed system at a given. As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Vapour. What Is Absolute Vapour Pressure.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Absolute Vapour Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); That is, the vapour pressure resulting from a liquid (or solid) evaporation above a liquid. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT 6 Gases PowerPoint Presentation, free download ID352441 What Is Absolute Vapour Pressure Vapor pressures are dependent only on temperature and nothing else. The pressure exerted by the vapor in equilibrium with a liquid in a closed container at a given temperature is called the liquid’s vapor. A liquid’s vapour pressure is a vapour’s equilibrium pressure above its liquid (or solid); Vapor pressure is the pressure exerted by a vapor that is in. What Is Absolute Vapour Pressure.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation ID1459744 What Is Absolute Vapour Pressure As the temperature of a liquid increases, the vapor pressure of the liquid increases until it equals the external pressure, or the atmospheric pressure in the case of an open container. Vapour pressure is the pressure exerted by the vapour of a substance in thermodynamic equilibrium with its condensed. Vapor pressure is the pressure exerted by a vapor that is. What Is Absolute Vapour Pressure.