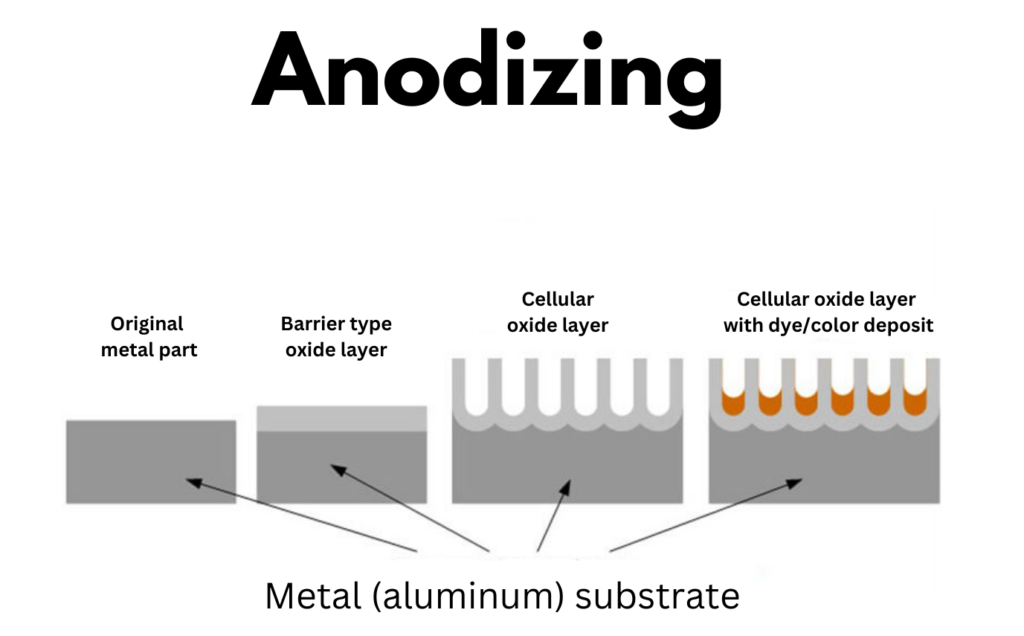
Anodizing Aluminum Without Sulfuric Acid . But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. A current is passed through the part, causing negatively. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. The anodizing and dying of aluminum parts at home has long been a popular project for many people.
from www.valencesurfacetech.com
But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. The anodizing and dying of aluminum parts at home has long been a popular project for many people. A current is passed through the part, causing negatively. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability.
Anodizing Vs. Plating Key Differences And Similarities
Anodizing Aluminum Without Sulfuric Acid The anodizing and dying of aluminum parts at home has long been a popular project for many people. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. The anodizing and dying of aluminum parts at home has long been a popular project for many people. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. A current is passed through the part, causing negatively.
From fractory.com
Aluminium Anodising Process & Benefits Explained Fractory Anodizing Aluminum Without Sulfuric Acid Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. The anodizing and dying of aluminum parts at home has long been a popular project for many people. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Also known as sulfuric acid anodizing, type ii anodized. Anodizing Aluminum Without Sulfuric Acid.
From cherellesmalley.blogspot.com
diy anodizing aluminum without sulfuric acid Cherelle Smalley Anodizing Aluminum Without Sulfuric Acid Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. It creates a thicker oxide layer. Anodizing Aluminum Without Sulfuric Acid.
From www.researchgate.net
SEM images showing the morphology of the anodizing aluminum alloy Anodizing Aluminum Without Sulfuric Acid When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. A current is passed through the part, causing negatively. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Anodizing may seem daunting, but with practice and the right approach, you too can. Anodizing Aluminum Without Sulfuric Acid.
From www.mdpi.com
Coatings Free FullText A Review on Anodizing of Aerospace Aluminum Anodizing Aluminum Without Sulfuric Acid Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. The anodizing and dying of aluminum parts at home has long been a popular project for many people. It creates a thicker oxide layer (0.0002 to 0.001 inches). Anodizing Aluminum Without Sulfuric Acid.
From some-disassembly-required.com
Anodizing Aluminum Without Sulfuric Acid Some Disassembly Required Anodizing Aluminum Without Sulfuric Acid A current is passed through the part, causing negatively. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. The anodizing and dying of aluminum parts at home has long been a popular project for many people. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion. Anodizing Aluminum Without Sulfuric Acid.
From www.semanticscholar.org
Figure 2 from The Effect of Ozonizing Sulfuric Acid Electrolyte with or Anodizing Aluminum Without Sulfuric Acid When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. A current is passed through the part, causing negatively. Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly. Anodizing Aluminum Without Sulfuric Acid.
From some-disassembly-required.com
Anodizing Aluminum Without Sulfuric Acid Some Disassembly Required Anodizing Aluminum Without Sulfuric Acid It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. A current is passed through the part, causing negatively. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. Anodizing. Anodizing Aluminum Without Sulfuric Acid.
From www.aluminumanodizing.com
Hard Anodizing Companies Hard Anodizing Services Anodizing Aluminum Without Sulfuric Acid A current is passed through the part, causing negatively. The anodizing and dying of aluminum parts at home has long been a popular project for many people. When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. Also known as sulfuric acid anodizing, type ii anodized aluminum. Anodizing Aluminum Without Sulfuric Acid.
From leadrp.net
Black Anodized Aluminum Basics Understanding Black Anodizing Process Anodizing Aluminum Without Sulfuric Acid Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Also known as sulfuric acid anodizing, type ii. Anodizing Aluminum Without Sulfuric Acid.
From fineartamerica.com
About Us Indaco Coats Aluminium and Titanium Anodizing Photograph by Anodizing Aluminum Without Sulfuric Acid When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. A current is passed through the part, causing negatively. Aluminum anodizing is an electrolytic process that takes place. Anodizing Aluminum Without Sulfuric Acid.
From anodizedaluminumdzutsugetsu.blogspot.com
Anodized Aluminum Anodized Aluminum Melting Point Anodizing Aluminum Without Sulfuric Acid Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. A current is passed through the part, causing negatively. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better. Anodizing Aluminum Without Sulfuric Acid.
From some-disassembly-required.com
Anodizing Aluminum Without Sulfuric Acid Some Disassembly Required Anodizing Aluminum Without Sulfuric Acid It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. Anodizing may seem daunting, but with practice and the right. Anodizing Aluminum Without Sulfuric Acid.
From aluconsult.com
How to Anodize Aluminum Type II Anodizing for Beginners AluConsult Anodizing Aluminum Without Sulfuric Acid Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. The anodizing and dying of aluminum parts at home has. Anodizing Aluminum Without Sulfuric Acid.
From some-disassembly-required.com
Anodizing Aluminum Without Sulfuric Acid Some Disassembly Required Anodizing Aluminum Without Sulfuric Acid When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. The anodizing and dying of aluminum parts at home has long been a popular project for many people. Aluminum anodizing is an electrolytic. Anodizing Aluminum Without Sulfuric Acid.
From www.researchgate.net
SEM images showing the morphology of the anodizing aluminum alloy Anodizing Aluminum Without Sulfuric Acid But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. A current is passed through the part, causing. Anodizing Aluminum Without Sulfuric Acid.
From www.valencesurfacetech.com
Anodizing Aluminum Types, Benefits, And Process Explained Anodizing Aluminum Without Sulfuric Acid But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. When paired with the proper dc current along with a. Anodizing Aluminum Without Sulfuric Acid.
From mintaphan.blogspot.com
diy anodizing aluminum without sulfuric acid Minta Phan Anodizing Aluminum Without Sulfuric Acid A current is passed through the part, causing negatively. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern.. Anodizing Aluminum Without Sulfuric Acid.
From www.researchgate.net
SEM images showing the morphology of the anodizing aluminum alloy Anodizing Aluminum Without Sulfuric Acid The anodizing and dying of aluminum parts at home has long been a popular project for many people. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. When paired with the proper dc current along with. Anodizing Aluminum Without Sulfuric Acid.
From some-disassembly-required.com
Anodizing Aluminum Without Sulfuric Acid Some Disassembly Required Anodizing Aluminum Without Sulfuric Acid Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. The anodizing and dying of aluminum parts at home has long been a popular project for many people. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. Anodizing may seem daunting, but. Anodizing Aluminum Without Sulfuric Acid.
From fractory.com
Aluminium Anodising Process & Benefits Explained Fractory Anodizing Aluminum Without Sulfuric Acid It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. The anodizing and dying of aluminum parts at home has long been a popular project for many people. A current is passed through the part, causing negatively. When paired with the proper dc current along with a cathode, the sodium bisulfate. Anodizing Aluminum Without Sulfuric Acid.
From some-disassembly-required.com
Anodizing Aluminum Without Sulfuric Acid Some Disassembly Required Anodizing Aluminum Without Sulfuric Acid Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Also known as sulfuric acid anodizing, type ii anodized aluminum. Anodizing Aluminum Without Sulfuric Acid.
From www.designedanodizing.co.za
Anodizing Explained Designed Anodizing Anodizing Aluminum Without Sulfuric Acid When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. Also. Anodizing Aluminum Without Sulfuric Acid.
From www.medicaldesignandoutsourcing.com
How anodizing can make aluminum suitable for reusable medical devices Anodizing Aluminum Without Sulfuric Acid Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. But. Anodizing Aluminum Without Sulfuric Acid.
From www.researchgate.net
SEM crosssectional micrographs of the samples anodized in sulfuric Anodizing Aluminum Without Sulfuric Acid When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization.. Anodizing Aluminum Without Sulfuric Acid.
From some-disassembly-required.com
Anodizing Aluminum Without Sulfuric Acid Some Disassembly Required Anodizing Aluminum Without Sulfuric Acid But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. A current is passed through the part, causing. Anodizing Aluminum Without Sulfuric Acid.
From www.mdpi.com
Coatings Free FullText A Review on Anodizing of Aerospace Aluminum Anodizing Aluminum Without Sulfuric Acid The anodizing and dying of aluminum parts at home has long been a popular project for many people. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. A current is passed through the part, causing negatively.. Anodizing Aluminum Without Sulfuric Acid.
From www.valencesurfacetech.com
Anodizing Vs. Plating Key Differences And Similarities Anodizing Aluminum Without Sulfuric Acid Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. A current is passed through the part, causing negatively. It creates. Anodizing Aluminum Without Sulfuric Acid.
From mintaphan.blogspot.com
diy anodizing aluminum without sulfuric acid Minta Phan Anodizing Aluminum Without Sulfuric Acid But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. Also known as sulfuric acid anodizing, type ii. Anodizing Aluminum Without Sulfuric Acid.
From jimbothelion.blogspot.com
Diy Anodizing Aluminum Without Sulfuric Acid / Anodizing And Dyeing Anodizing Aluminum Without Sulfuric Acid A current is passed through the part, causing negatively. Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. Aluminum anodizing is an electrolytic process that takes place in. Anodizing Aluminum Without Sulfuric Acid.
From unix-troubleshooting.blogspot.com
diy anodizing aluminum without sulfuric acid Journal Anodizing Aluminum Without Sulfuric Acid When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something.. Anodizing Aluminum Without Sulfuric Acid.
From some-disassembly-required.com
Anodizing Aluminum Without Sulfuric Acid Some Disassembly Required Anodizing Aluminum Without Sulfuric Acid Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Anodizing may seem daunting, but with practice and the right approach, you too can transform ordinary aluminum into something. When paired with the proper dc current along with. Anodizing Aluminum Without Sulfuric Acid.
From www.mdpi.com
Metals Free FullText Influence of the Anodizing Time on the Anodizing Aluminum Without Sulfuric Acid The anodizing and dying of aluminum parts at home has long been a popular project for many people. When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. A current. Anodizing Aluminum Without Sulfuric Acid.
From www.anodizingequipment.com
ALUMINUM & TITANIUM ANODIZING EQUIPMENT Anodizing Aluminum Without Sulfuric Acid Also known as sulfuric acid anodizing, type ii anodized aluminum is the most commonly used type of anodization. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. A current is passed through the part, causing negatively. When paired. Anodizing Aluminum Without Sulfuric Acid.
From www.theengineeringprojects.com
Everything You Need To Know About Anodizing Aluminum Colors The Anodizing Aluminum Without Sulfuric Acid The anodizing and dying of aluminum parts at home has long been a popular project for many people. But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. When paired with the proper dc current along with a cathode, the sodium bisulfate easily anodizes an aluminum workpiece and renders it ready. Also known as sulfuric acid. Anodizing Aluminum Without Sulfuric Acid.
From www.mdpi.com
Materials Free FullText Corrosion Resistance of Aluminum Alloy Anodizing Aluminum Without Sulfuric Acid But the use of battery acid, typically (29% to 32% sulfuric acid) causes concern. Aluminum anodizing is an electrolytic process that takes place in an electrolyte such as dilute sulfuric acid. A current is passed through the part, causing negatively. It creates a thicker oxide layer (0.0002 to 0.001 inches) than type i, providing better corrosion resistance and durability. The. Anodizing Aluminum Without Sulfuric Acid.