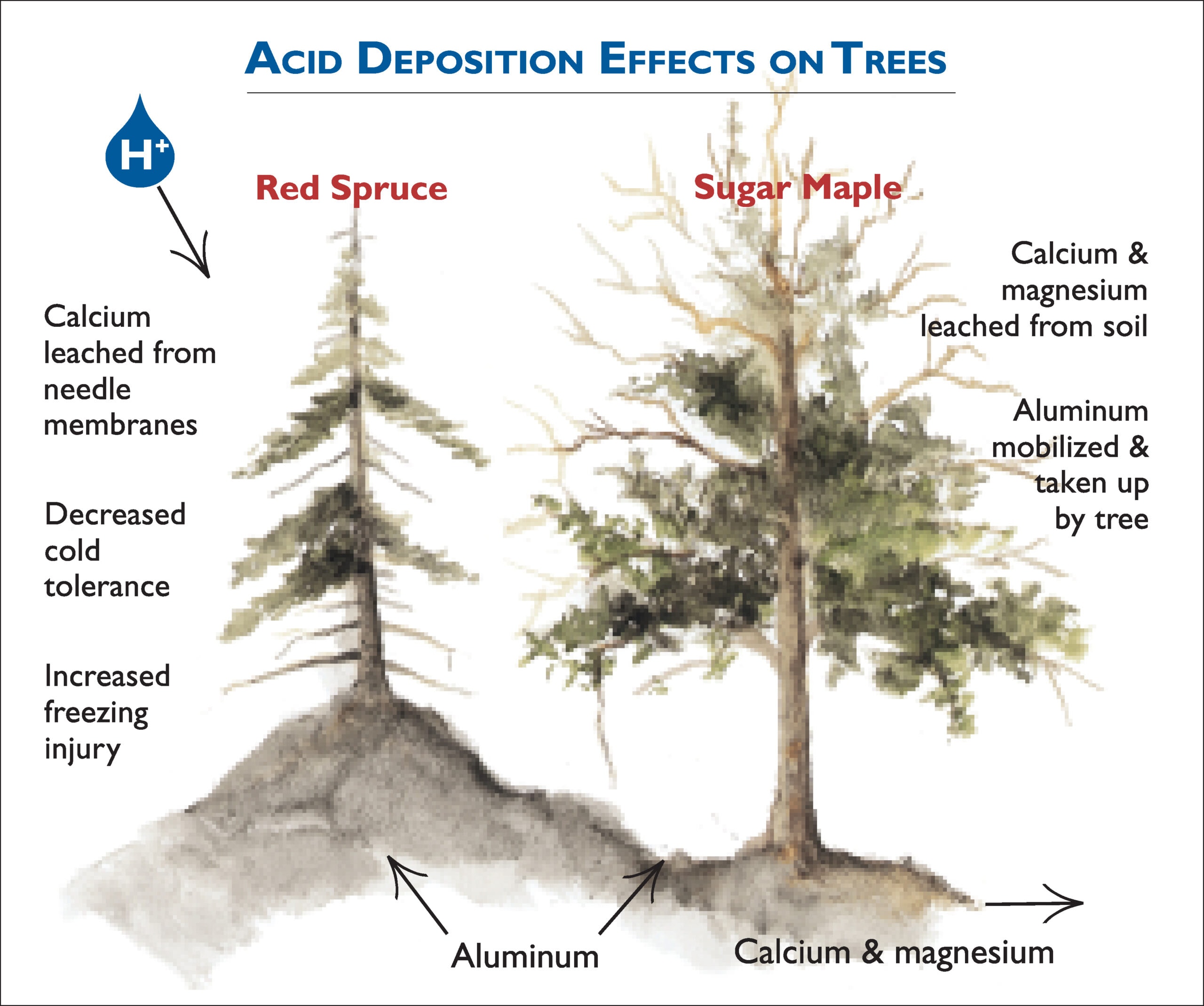
How Does Acid Affect Limestone . When sulfurous, sulfuric, and nitric acids in polluted air and rain. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Weathering is the breakdown of rock by physical, chemical or biological processes. Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. How does acid precipitation affect marble and limestone buildings? It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. Acid precipitation affects stone primarily in two ways: Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. This causes the limestone to dissolve. Limestone is mostly made up of the mineral calcium carbonate (caco3). This is not very soluble, so rocks don't dissolve very quickly. Often, acidic groundwater is the culprit;
from ar.inspiredpencil.com
Weathering is the breakdown of rock by physical, chemical or biological processes. It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. How does acid precipitation affect marble and limestone buildings? Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. Limestone is mostly made up of the mineral calcium carbonate (caco3). This causes the limestone to dissolve. Acid precipitation affects stone primarily in two ways: Often, acidic groundwater is the culprit; When sulfurous, sulfuric, and nitric acids in polluted air and rain. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the.
Acid Rain Before And After
How Does Acid Affect Limestone When sulfurous, sulfuric, and nitric acids in polluted air and rain. Weathering is the breakdown of rock by physical, chemical or biological processes. When sulfurous, sulfuric, and nitric acids in polluted air and rain. Limestone is mostly made up of the mineral calcium carbonate (caco3). Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. This causes the limestone to dissolve. This is not very soluble, so rocks don't dissolve very quickly. Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. Acid precipitation affects stone primarily in two ways: Often, acidic groundwater is the culprit; How does acid precipitation affect marble and limestone buildings? When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the.
From exovylano.blob.core.windows.net
How Does Acid Precipitation Affect Ecosystems at Hazel Barksdale blog How Does Acid Affect Limestone Weathering is the breakdown of rock by physical, chemical or biological processes. When sulfurous, sulfuric, and nitric acids in polluted air and rain. Limestone is mostly made up of the mineral calcium carbonate (caco3). Often, acidic groundwater is the culprit; Acid precipitation affects stone primarily in two ways: Limestone areas are predominantly affected by chemical weathering when rainwater, which contains. How Does Acid Affect Limestone.
From edu.rsc.org
What are the effects of acid rain? 1114 years Lesson plan RSC How Does Acid Affect Limestone This causes the limestone to dissolve. Often, acidic groundwater is the culprit; Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. How does acid precipitation affect marble and limestone buildings? Weathering is. How Does Acid Affect Limestone.
From www.studypool.com
SOLUTION How does changing the concentration of acid affect the rate How Does Acid Affect Limestone Acid precipitation affects stone primarily in two ways: Limestone is mostly made up of the mineral calcium carbonate (caco3). This causes the limestone to dissolve. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground. How Does Acid Affect Limestone.
From ar.inspiredpencil.com
Limestone Building Acid Rain How Does Acid Affect Limestone Often, acidic groundwater is the culprit; It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. Weathering is the breakdown of rock by physical, chemical or biological processes. Acid precipitation affects stone primarily in two ways: Limestone is mostly made up of the mineral calcium carbonate (caco3). Limestone areas. How Does Acid Affect Limestone.
From earth.org
How Does Acid Rain Affect the Environment How Does Acid Affect Limestone Limestone is mostly made up of the mineral calcium carbonate (caco3). When sulfurous, sulfuric, and nitric acids in polluted air and rain. How does acid precipitation affect marble and limestone buildings? This is not very soluble, so rocks don't dissolve very quickly. Acid precipitation affects stone primarily in two ways: Often, acidic groundwater is the culprit; This causes the limestone. How Does Acid Affect Limestone.
From www.sciencephoto.com
Reaction of limestone and acid Stock Image A500/0760 Science How Does Acid Affect Limestone Weathering is the breakdown of rock by physical, chemical or biological processes. How does acid precipitation affect marble and limestone buildings? Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. This causes. How Does Acid Affect Limestone.
From www.thenakedscientists.com
What happens when acid reacts with limestone? Science Questions How Does Acid Affect Limestone Often, acidic groundwater is the culprit; How does acid precipitation affect marble and limestone buildings? Acid precipitation affects stone primarily in two ways: When sulfurous, sulfuric, and nitric acids in polluted air and rain. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. This is not very soluble, so rocks. How Does Acid Affect Limestone.
From www.dreamstime.com
Differential Solution of a Limestone Rock Stock Image Image of How Does Acid Affect Limestone Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. How does acid precipitation affect marble and limestone buildings? Weathering is the breakdown of rock by physical, chemical or biological processes. Limestone is. How Does Acid Affect Limestone.
From www.sciencephoto.com
Limestoneacid reaction Stock Image C040/2671 Science Photo Library How Does Acid Affect Limestone This causes the limestone to dissolve. Weathering is the breakdown of rock by physical, chemical or biological processes. Acid precipitation affects stone primarily in two ways: How does acid precipitation affect marble and limestone buildings? It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. When sulfurous, sulfuric, and. How Does Acid Affect Limestone.
From slideplayer.com
Neutralising Acids with Limestone ppt download How Does Acid Affect Limestone This causes the limestone to dissolve. This is not very soluble, so rocks don't dissolve very quickly. How does acid precipitation affect marble and limestone buildings? It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. Acid rain that seeps into the subsurface through soil penetration or stream loss. How Does Acid Affect Limestone.
From www.studypool.com
SOLUTION How increased concentrations of acid affect the rate of How Does Acid Affect Limestone Acid precipitation affects stone primarily in two ways: Weathering is the breakdown of rock by physical, chemical or biological processes. This causes the limestone to dissolve. How does acid precipitation affect marble and limestone buildings? Often, acidic groundwater is the culprit; Limestone is mostly made up of the mineral calcium carbonate (caco3). Acid rain that seeps into the subsurface through. How Does Acid Affect Limestone.
From www.coursehero.com
[Solved] How does acid and alkaline affect white vegetable pigment How Does Acid Affect Limestone How does acid precipitation affect marble and limestone buildings? It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. Limestone is mostly made up of the mineral calcium carbonate (caco3). Often,. How Does Acid Affect Limestone.
From www.internetgeography.net
How does weathering affect limestone? Geography How Does Acid Affect Limestone Limestone is mostly made up of the mineral calcium carbonate (caco3). Weathering is the breakdown of rock by physical, chemical or biological processes. This causes the limestone to dissolve. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. When sulfurous, sulfuric, and nitric acids in polluted air and rain. Acid. How Does Acid Affect Limestone.
From fineartamerica.com
Limestone Reacting With Acid Photograph by Science Photo Library Fine How Does Acid Affect Limestone When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Limestone is mostly made up of the mineral calcium carbonate (caco3). Acid precipitation affects stone primarily in two ways: This is not very soluble, so rocks don't dissolve very quickly. How does acid precipitation affect marble and limestone buildings? When sulfurous, sulfuric,. How Does Acid Affect Limestone.
From letstalkgeography.com
How does Acid Rain affect Climate change? Let's Talk Geography How Does Acid Affect Limestone Limestone is mostly made up of the mineral calcium carbonate (caco3). This is not very soluble, so rocks don't dissolve very quickly. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. How does acid precipitation affect marble and limestone buildings? Often, acidic groundwater is the culprit; This causes the limestone. How Does Acid Affect Limestone.
From www.tehrantimes.com
And now comes the acid rain Tehran Times How Does Acid Affect Limestone Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. Often, acidic groundwater is the culprit; Limestone is mostly made up of the mineral calcium carbonate (caco3). When sulfurous, sulfuric, and nitric acids. How Does Acid Affect Limestone.
From www.pinterest.com
Cover seashells with vinegar and How Does Acid Affect Limestone Acid precipitation affects stone primarily in two ways: How does acid precipitation affect marble and limestone buildings? It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. Often, acidic groundwater is the culprit; Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground. How Does Acid Affect Limestone.
From www.studypool.com
SOLUTION How acid rain affects limestone buildings Studypool How Does Acid Affect Limestone Limestone is mostly made up of the mineral calcium carbonate (caco3). This is not very soluble, so rocks don't dissolve very quickly. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. When sulfurous, sulfuric, and nitric acids in polluted air and rain. Acid precipitation affects stone primarily in two ways:. How Does Acid Affect Limestone.
From www.scienceshorts.com
How Does Limestone Protect Lakes from Acid Rain How Does Acid Affect Limestone When sulfurous, sulfuric, and nitric acids in polluted air and rain. It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Acid rain that seeps into the subsurface through soil penetration. How Does Acid Affect Limestone.
From zh.mindat.org
Limestone Mineral information, data and localities. How Does Acid Affect Limestone Weathering is the breakdown of rock by physical, chemical or biological processes. This is not very soluble, so rocks don't dissolve very quickly. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. When sulfurous, sulfuric, and nitric acids in polluted air and rain. It can dissolve supportive calcium carbonate in. How Does Acid Affect Limestone.
From www.studypool.com
SOLUTION How acid rain affects limestone buildings Studypool How Does Acid Affect Limestone It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. Acid precipitation. How Does Acid Affect Limestone.
From socratic.org
How does limestone change throughout the rock cycle? Socratic How Does Acid Affect Limestone Acid precipitation affects stone primarily in two ways: Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed. How Does Acid Affect Limestone.
From giobeuaqs.blob.core.windows.net
Is Acid Rain Useful Or Destructive To The Environment at Martha Ganley blog How Does Acid Affect Limestone This causes the limestone to dissolve. Limestone is mostly made up of the mineral calcium carbonate (caco3). Acid precipitation affects stone primarily in two ways: Weathering is the breakdown of rock by physical, chemical or biological processes. When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Often, acidic groundwater is the. How Does Acid Affect Limestone.
From sciencing.com
Why Does Vinegar Affect Limestone? Sciencing How Does Acid Affect Limestone Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. Limestone is mostly made up of the mineral calcium carbonate (caco3). Acid precipitation affects stone primarily in two ways: Often, acidic groundwater is the culprit; This is not very soluble, so rocks don't dissolve very quickly. This causes the limestone to dissolve.. How Does Acid Affect Limestone.
From www.ehow.com
What Does Acid Rain Do to Limestone? ehow How Does Acid Affect Limestone Limestone is mostly made up of the mineral calcium carbonate (caco3). Often, acidic groundwater is the culprit; How does acid precipitation affect marble and limestone buildings? This is not very soluble, so rocks don't dissolve very quickly. Weathering is the breakdown of rock by physical, chemical or biological processes. Limestone areas are predominantly affected by chemical weathering when rainwater, which. How Does Acid Affect Limestone.
From www.numerade.com
SOLVEDWrite reactions to show how the nitric and sulfuric acids in How Does Acid Affect Limestone When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Often, acidic groundwater is the culprit; Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. When sulfurous, sulfuric, and nitric acids in polluted air and rain. Acid precipitation affects stone primarily in. How Does Acid Affect Limestone.
From brainly.in
Write the mechanism of acid rain? what is marble cancer? how is it How Does Acid Affect Limestone How does acid precipitation affect marble and limestone buildings? When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid, reacts with limestone. It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets. How Does Acid Affect Limestone.
From www.dreamstime.com
Limestone Surface Eroded by Acid Rain. Stock Image Image of material How Does Acid Affect Limestone It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. How does acid precipitation affect marble and limestone buildings? Acid precipitation affects stone primarily in two ways: Limestone is mostly made. How Does Acid Affect Limestone.
From ar.inspiredpencil.com
Acid Rain Before And After How Does Acid Affect Limestone Weathering is the breakdown of rock by physical, chemical or biological processes. It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. Limestone areas are predominantly affected by chemical weathering when. How Does Acid Affect Limestone.
From www.pmsc.com.ph
Limestone PMSC How Does Acid Affect Limestone Limestone is mostly made up of the mineral calcium carbonate (caco3). When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. Often, acidic groundwater is the culprit; When sulfurous, sulfuric, and nitric acids in polluted air and rain. Weathering is the breakdown of rock by physical, chemical or biological processes. Limestone areas. How Does Acid Affect Limestone.
From www.studypool.com
SOLUTION Discover how certain factors can affect the rate of reaction How Does Acid Affect Limestone Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. Limestone is mostly made up of the mineral calcium carbonate (caco3). When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. How does acid precipitation affect marble and limestone buildings? Often, acidic groundwater is. How Does Acid Affect Limestone.
From fineartamerica.com
Limestone In Acid Photograph by David Taylor/science Photo Library How Does Acid Affect Limestone When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. How does acid precipitation affect marble and limestone buildings? Often, acidic groundwater is the culprit; Limestone is mostly made up of the mineral calcium carbonate (caco3). Limestone areas are predominantly affected by chemical weathering when rainwater, which contains a weak carbonic acid,. How Does Acid Affect Limestone.
From www.vrogue.co
Know The Basic Behind Acid Rain And Harmful Effects vrogue.co How Does Acid Affect Limestone When sulfurous, sulfuric, and nitric acids in polluted air react with the calcite in marble and limestone, the. How does acid precipitation affect marble and limestone buildings? It can dissolve supportive calcium carbonate in a layer of limestone rock, which gets washed away through the process of erosion. This is not very soluble, so rocks don't dissolve very quickly. When. How Does Acid Affect Limestone.
From fineartamerica.com
Limestone Reacting With Acid Photograph by Andrew Lambert Photography How Does Acid Affect Limestone This is not very soluble, so rocks don't dissolve very quickly. Acid precipitation affects stone primarily in two ways: Weathering is the breakdown of rock by physical, chemical or biological processes. Often, acidic groundwater is the culprit; Acid rain that seeps into the subsurface through soil penetration or stream loss can dissolve underground limestone rocks. When sulfurous, sulfuric, and nitric. How Does Acid Affect Limestone.
From www.studypool.com
SOLUTION How does changing the concentration of acid affect the rate How Does Acid Affect Limestone Often, acidic groundwater is the culprit; When sulfurous, sulfuric, and nitric acids in polluted air and rain. Weathering is the breakdown of rock by physical, chemical or biological processes. Acid precipitation affects stone primarily in two ways: How does acid precipitation affect marble and limestone buildings? This is not very soluble, so rocks don't dissolve very quickly. This causes the. How Does Acid Affect Limestone.