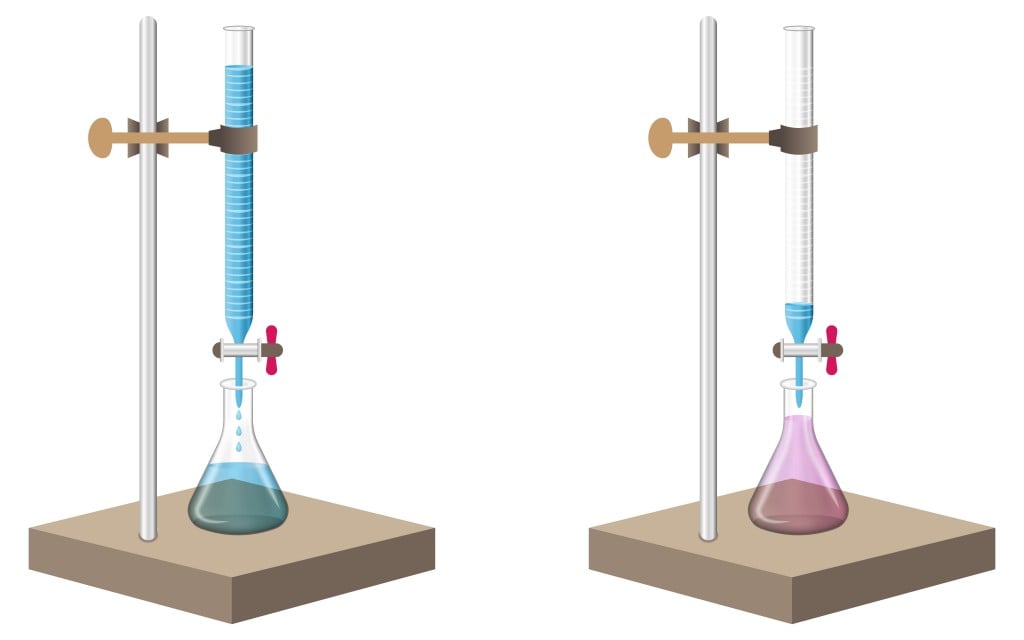
Chemistry Titration Neutralization . The first will be the titration of an acid by a base. We will show two examples of a titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. In a titration, an acid or a base is in a flask or a beaker. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react. neutralization titrations are widely used to determine the amounts of acids and bases and to monitor the progress of reactions. The second will be the titration of a base by an acid. One of the most common and widely used ways to complete a neutralization reaction is through titration.
from www.scienceabc.com
neutralization titrations are widely used to determine the amounts of acids and bases and to monitor the progress of reactions. One of the most common and widely used ways to complete a neutralization reaction is through titration. The second will be the titration of a base by an acid. We will show two examples of a titration. The first will be the titration of an acid by a base. In a titration, an acid or a base is in a flask or a beaker. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react.
Titration Chemistry Definition, Explanation, Formula And Calculation
Chemistry Titration Neutralization We will show two examples of a titration. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. The second will be the titration of a base by an acid. The first will be the titration of an acid by a base. One of the most common and widely used ways to complete a neutralization reaction is through titration. We will show two examples of a titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. neutralization titrations are widely used to determine the amounts of acids and bases and to monitor the progress of reactions. In a titration, an acid or a base is in a flask or a beaker. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react.
From www.priyamstudycentre.com
Acid Base Titration Principle, Types, Process, Indicators Chemistry Titration Neutralization It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. The first will be the titration of an acid by a base. The second will be the titration of a base by an acid. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in. Chemistry Titration Neutralization.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Chemistry Titration Neutralization neutralization titrations are widely used to determine the amounts of acids and bases and to monitor the progress of reactions. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. titration is the slow addition of one solution of a known concentration (called a titrant) to. Chemistry Titration Neutralization.
From www.studypool.com
SOLUTION Analytical chemistry notes on 5 principles of neutralization Chemistry Titration Neutralization The second will be the titration of a base by an acid. neutralization titrations are widely used to determine the amounts of acids and bases and to monitor the progress of reactions. One of the most common and widely used ways to complete a neutralization reaction is through titration. We will show two examples of a titration. in. Chemistry Titration Neutralization.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Chemistry Titration Neutralization neutralization titrations are widely used to determine the amounts of acids and bases and to monitor the progress of reactions. One of the most common and widely used ways to complete a neutralization reaction is through titration. We will show two examples of a titration. titration is the slow addition of one solution of a known concentration (called. Chemistry Titration Neutralization.
From www.istockphoto.com
Acidbase Titration And Neutralization Reaction In Chemistry Stock Chemistry Titration Neutralization in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. The first will be the titration of an acid by a base. One of the most common and. Chemistry Titration Neutralization.
From www.scribd.com
7 Neutralization Titrations Titration Chemistry Chemistry Titration Neutralization It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. In a titration, an acid or a base is in a flask or a beaker. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Chemistry Titration Neutralization.
From www.teachoo.com
Neutralization Reaction Definition, Equation and Examples Teachoo Chemistry Titration Neutralization titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react. neutralization titrations are widely used to determine the amounts of acids and bases and to. Chemistry Titration Neutralization.
From slidetodoc.com
Titration and AcidBase Neutralization Chemistry Mrs Coyle Acid Chemistry Titration Neutralization It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. In a titration, an acid or a base is in a flask or a beaker. We will show two examples of a titration. One of the most common and widely used ways to complete a neutralization reaction is. Chemistry Titration Neutralization.
From vdocuments.mx
Acids and Bases Titrations AP Chemistry. Neutralization Reactions and Chemistry Titration Neutralization In a titration, an acid or a base is in a flask or a beaker. We will show two examples of a titration. The first will be the titration of an acid by a base. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. in chemistry,. Chemistry Titration Neutralization.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Chemistry Titration Neutralization It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. The second will be the titration of a base by an acid. One of the most common and widely used ways to complete a neutralization reaction is through titration. titration is the slow addition of one solution. Chemistry Titration Neutralization.
From www.scribd.com
Anachem Neutralization PDF Chemistry Titration Chemistry Titration Neutralization One of the most common and widely used ways to complete a neutralization reaction is through titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with. Chemistry Titration Neutralization.
From www.studypool.com
SOLUTION Analytical chemistry notes on 5 principles of neutralization Chemistry Titration Neutralization The second will be the titration of a base by an acid. The first will be the titration of an acid by a base. In a titration, an acid or a base is in a flask or a beaker. One of the most common and widely used ways to complete a neutralization reaction is through titration. It is the method. Chemistry Titration Neutralization.
From www.youtube.com
Chemistry 30 Acid Base Neutralization and Titration YouTube Chemistry Titration Neutralization One of the most common and widely used ways to complete a neutralization reaction is through titration. The first will be the titration of an acid by a base. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. in chemistry, neutralization or neutralisation (see. Chemistry Titration Neutralization.
From materiallibrarysevert.z21.web.core.windows.net
Neutralization And Titration Worksheet Chemistry Titration Neutralization One of the most common and widely used ways to complete a neutralization reaction is through titration. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react. The first will be the titration of an acid by a base. In a titration, an acid or a base is in a. Chemistry Titration Neutralization.
From www.tes.com
Acid Base Titrations, Neutralization, Equivalence Point Grade 11 Chemistry Titration Neutralization One of the most common and widely used ways to complete a neutralization reaction is through titration. In a titration, an acid or a base is in a flask or a beaker. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. neutralization titrations are widely used. Chemistry Titration Neutralization.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Chemistry Titration Neutralization One of the most common and widely used ways to complete a neutralization reaction is through titration. The second will be the titration of a base by an acid. In a titration, an acid or a base is in a flask or a beaker. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid. Chemistry Titration Neutralization.
From www.scribd.com
Week 6b Neutralization PDF Titration Chemistry Chemistry Titration Neutralization The first will be the titration of an acid by a base. In a titration, an acid or a base is in a flask or a beaker. neutralization titrations are widely used to determine the amounts of acids and bases and to monitor the progress of reactions. It is the method of finding the concentration of an unknown acid/base. Chemistry Titration Neutralization.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Chemistry Titration Neutralization It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. One of the most common and widely used ways to complete a neutralization reaction is through titration. The second will be the titration of a base by an acid. We will show two examples of a titration. The. Chemistry Titration Neutralization.
From thechemistrynotes.com
Acidbase Titration 4 Types, Theory, Working Principle Chemistry Titration Neutralization The second will be the titration of a base by an acid. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. In a titration, an acid or a base is in a flask or a beaker. in chemistry, neutralization or neutralisation (see spelling differences) is a. Chemistry Titration Neutralization.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Chemistry Titration Neutralization One of the most common and widely used ways to complete a neutralization reaction is through titration. The first will be the titration of an acid by a base. We will show two examples of a titration. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. In. Chemistry Titration Neutralization.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Chemistry Titration Neutralization One of the most common and widely used ways to complete a neutralization reaction is through titration. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution. Chemistry Titration Neutralization.
From www.scribd.com
Principles of Neutralization Titration PDF Titration Chemistry Chemistry Titration Neutralization It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. We will show two examples of a titration. neutralization titrations are widely used to determine the amounts of acids and bases and to monitor the progress of reactions. in chemistry, neutralization or neutralisation (see spelling differences). Chemistry Titration Neutralization.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Chemistry Titration Neutralization It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. neutralization titrations are widely used to determine the amounts of acids and bases and to monitor the progress of reactions. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and. Chemistry Titration Neutralization.
From tr.scribd.com
Application of Neutralization Titrations PDF Titration Chemistry Chemistry Titration Neutralization One of the most common and widely used ways to complete a neutralization reaction is through titration. In a titration, an acid or a base is in a flask or a beaker. The first will be the titration of an acid by a base. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid. Chemistry Titration Neutralization.
From www.chemicals.co.uk
What is Neutralisation in Chemistry? The Chemistry Blog Chemistry Titration Neutralization We will show two examples of a titration. One of the most common and widely used ways to complete a neutralization reaction is through titration. neutralization titrations are widely used to determine the amounts of acids and bases and to monitor the progress of reactions. titration is the slow addition of one solution of a known concentration (called. Chemistry Titration Neutralization.
From relationshipbetween.com
Difference Between Titration And Neutralization Relationship Between Chemistry Titration Neutralization In a titration, an acid or a base is in a flask or a beaker. We will show two examples of a titration. One of the most common and widely used ways to complete a neutralization reaction is through titration. The first will be the titration of an acid by a base. The second will be the titration of a. Chemistry Titration Neutralization.
From www.studypool.com
SOLUTION Acid Base Titration (Neutralization Titration) Studypool Chemistry Titration Neutralization One of the most common and widely used ways to complete a neutralization reaction is through titration. The first will be the titration of an acid by a base. neutralization titrations are widely used to determine the amounts of acids and bases and to monitor the progress of reactions. We will show two examples of a titration. titration. Chemistry Titration Neutralization.
From www.youtube.com
Neutralization curve_Acidbase titration curve YouTube Chemistry Titration Neutralization We will show two examples of a titration. One of the most common and widely used ways to complete a neutralization reaction is through titration. The second will be the titration of a base by an acid. In a titration, an acid or a base is in a flask or a beaker. It is the method of finding the concentration. Chemistry Titration Neutralization.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Chemistry Titration Neutralization In a titration, an acid or a base is in a flask or a beaker. The first will be the titration of an acid by a base. We will show two examples of a titration. titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of. . Chemistry Titration Neutralization.
From chem.libretexts.org
17.4 Neutralization Reactions and Titration Curves Chemistry LibreTexts Chemistry Titration Neutralization The second will be the titration of a base by an acid. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. We will show two examples of a titration. One of the most common and widely used ways to complete a neutralization reaction is through titration. . Chemistry Titration Neutralization.
From study.com
Neutralization Reaction Definition, Equation & Examples Lesson Chemistry Titration Neutralization It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. In a titration, an acid or a base is in a flask or a beaker. The first will be the titration of an acid by a base. titration is the slow addition of one solution of a. Chemistry Titration Neutralization.
From www.studocu.com
Titration and neutralization wkst Chemistry 40S WAEC Titration and Chemistry Titration Neutralization in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react. We will show two examples of a titration. The second will be the titration of a base by an acid. The first will be the titration of an acid by a base. neutralization titrations are widely used to determine. Chemistry Titration Neutralization.
From www.studocu.com
Chemistry example 14 Principles of Neutralization Titrations 14A Chemistry Titration Neutralization It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. in chemistry, neutralization or neutralisation (see spelling differences) is a chemical reaction in which acid and a base react. The first will be the titration of an acid by a base. One of the most common and. Chemistry Titration Neutralization.
From www.differencebetween.com
Difference Between Titration and Neutralization Compare the Chemistry Titration Neutralization We will show two examples of a titration. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. One of the most common and widely used ways to complete a neutralization reaction is through titration. titration is the slow addition of one solution of a known concentration. Chemistry Titration Neutralization.
From www.studypool.com
SOLUTION Neutralization titrations strong acid strong base titration Chemistry Titration Neutralization In a titration, an acid or a base is in a flask or a beaker. It is the method of finding the concentration of an unknown acid/base by titrating it against a base/acid with a known concentration. We will show two examples of a titration. The second will be the titration of a base by an acid. in chemistry,. Chemistry Titration Neutralization.