What Are Catalysts In Chemistry . Learn how catalysts work, why they are important and what types of. This process is called catalysis. Catalysts provide alternative reaction pathways. Catalysts are conventionally divided into two categories: A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed.
from www.youtube.com
A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. Catalysts provide alternative reaction pathways. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. This process is called catalysis. Learn how catalysts work, why they are important and what types of. Catalysts are conventionally divided into two categories:
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube
What Are Catalysts In Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Learn how catalysts work, why they are important and what types of. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts are conventionally divided into two categories: This process is called catalysis. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts provide alternative reaction pathways.
From studymind.co.uk
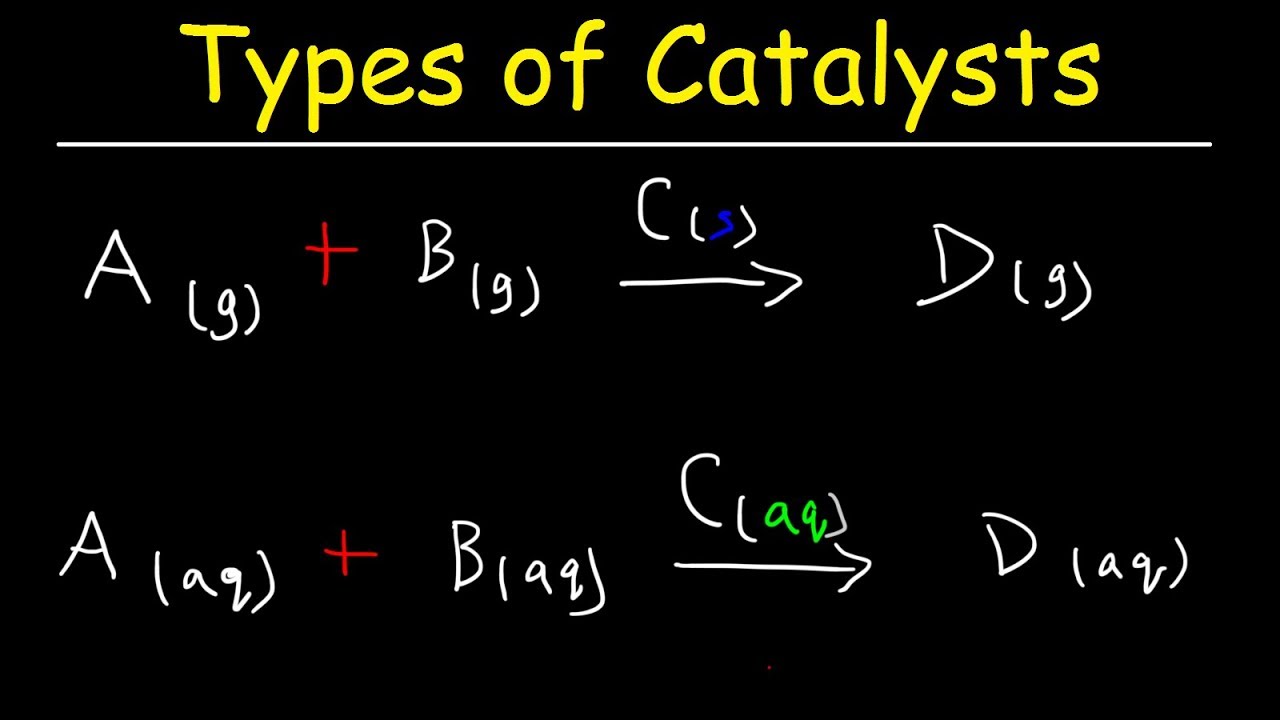
Catalysts (GCSE Chemistry) Study Mind What Are Catalysts In Chemistry A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. Catalysts are conventionally divided into two categories: This process is called catalysis.. What Are Catalysts In Chemistry.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation What Are Catalysts In Chemistry A catalyst is a material that speeds up chemical reactions by lowering the activation energy. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts provide alternative reaction pathways. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. In heterogeneous catalysis,. What Are Catalysts In Chemistry.
From www.thoughtco.com
Catalysis Definition in Chemistry What Are Catalysts In Chemistry This process is called catalysis. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts provide alternative reaction pathways. In heterogeneous catalysis, catalysts provide a surface to. Learn how catalysts work, why they are important and what types of. A catalyst is another substance. What Are Catalysts In Chemistry.
From www.mdpi.com
Catalysts Free FullText General and Prospective Views on Oxidation What Are Catalysts In Chemistry Learn how catalysts work, why they are important and what types of. Catalysts are conventionally divided into two categories: A catalyst is a material that speeds up chemical reactions by lowering the activation energy. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. In. What Are Catalysts In Chemistry.
From scitechdaily.com
Scientists Can Now Design Single Atom Catalysts for Important Chemical What Are Catalysts In Chemistry A catalyst is a material that speeds up chemical reactions by lowering the activation energy. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A. What Are Catalysts In Chemistry.
From scitechdaily.com
Science Made Simple What Are Catalysts? What Are Catalysts In Chemistry Learn how catalysts work, why they are important and what types of. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts provide alternative reaction pathways. This process is called catalysis. Catalysts. What Are Catalysts In Chemistry.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste What Are Catalysts In Chemistry Catalysts provide alternative reaction pathways. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In heterogeneous catalysis, catalysts provide a surface to. This process is called catalysis. A. What Are Catalysts In Chemistry.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 What Are Catalysts In Chemistry A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts are conventionally divided into two categories: Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This process is called catalysis. In heterogeneous catalysis, catalysts provide a surface to. Catalysts allow a reaction to proceed. What Are Catalysts In Chemistry.
From www.youtube.com
Homogeneous vs Heterogeneous Catalysts Basic Introduction YouTube What Are Catalysts In Chemistry A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts are conventionally divided into two categories: A catalyst is not consumed by the reaction and. What Are Catalysts In Chemistry.
From www.youtube.com
What are catalysts Chemistry for All The Fuse School YouTube What Are Catalysts In Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. This process is called catalysis. Learn how catalysts work, why they are important and what types of. Catalysts are conventionally divided into two categories: Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A. What Are Catalysts In Chemistry.
From www.eurekalert.org
Catalysis Illustration [IMAGE] EurekAlert! Science News Releases What Are Catalysts In Chemistry Learn how catalysts work, why they are important and what types of. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysts are conventionally divided into two categories:. What Are Catalysts In Chemistry.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free What Are Catalysts In Chemistry Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. This process is called catalysis. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Catalyst, in chemistry, any substance that increases the rate of a. What Are Catalysts In Chemistry.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst What Are Catalysts In Chemistry Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is called catalysis. Catalysts provide alternative reaction pathways. A catalyst is another substance than. What Are Catalysts In Chemistry.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID1803655 What Are Catalysts In Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. Catalysts provide alternative reaction. What Are Catalysts In Chemistry.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica What Are Catalysts In Chemistry This process is called catalysis. Catalysts provide alternative reaction pathways. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a. What Are Catalysts In Chemistry.
From www.slideshare.net
Catalyst What Are Catalysts In Chemistry In heterogeneous catalysis, catalysts provide a surface to. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. This process is called catalysis. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts provide alternative reaction pathways. A catalyst is another substance. What Are Catalysts In Chemistry.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis What Are Catalysts In Chemistry This process is called catalysis. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Learn how catalysts work, why they are important and what types of. In heterogeneous catalysis, catalysts provide a surface to. Catalyst, in chemistry, any substance that increases the rate of. What Are Catalysts In Chemistry.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID What Are Catalysts In Chemistry In heterogeneous catalysis, catalysts provide a surface to. This process is called catalysis. Catalysts provide alternative reaction pathways. Learn how catalysts work, why they are important and what types of. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is not consumed by the reaction and it may. What Are Catalysts In Chemistry.
From studymind.co.uk
Catalysts (GCSE Chemistry) Study Mind What Are Catalysts In Chemistry A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Learn how catalysts work, why they are important and what types of. This process is called catalysis. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is a chemical substance that affects the rate. What Are Catalysts In Chemistry.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2684800 What Are Catalysts In Chemistry Catalysts are conventionally divided into two categories: A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is called catalysis. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a. What Are Catalysts In Chemistry.
From www.youtube.com
How To Identify The Intermediate & Catalyst In a Reaction Mechanism What Are Catalysts In Chemistry Learn how catalysts work, why they are important and what types of. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. In heterogeneous catalysis, catalysts provide a surface to. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy. What Are Catalysts In Chemistry.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B What Are Catalysts In Chemistry A catalyst is a material that speeds up chemical reactions by lowering the activation energy. In heterogeneous catalysis, catalysts provide a surface to. Learn how catalysts work, why they are important and what types of. Catalysts provide alternative reaction pathways. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical. What Are Catalysts In Chemistry.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube What Are Catalysts In Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. In heterogeneous catalysis, catalysts. What Are Catalysts In Chemistry.
From www.youtube.com
Types of catalysts AP Chemistry Khan Academy YouTube What Are Catalysts In Chemistry In heterogeneous catalysis, catalysts provide a surface to. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. This process is called catalysis. Catalysts provide alternative reaction pathways. A catalyst. What Are Catalysts In Chemistry.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii What Are Catalysts In Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. This process is called catalysis. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for. What Are Catalysts In Chemistry.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram What Are Catalysts In Chemistry A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. This process is called catalysis. Learn how catalysts work, why they are important and what types. What Are Catalysts In Chemistry.
From www.youtube.com
Identifying catalysts in a reaction YouTube What Are Catalysts In Chemistry Catalysts are conventionally divided into two categories: A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is. What Are Catalysts In Chemistry.
From www.researchgate.net
Use of heterogeneous catalysis in various chemistry sectors Download What Are Catalysts In Chemistry A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. This process is called catalysis. In heterogeneous catalysis, catalysts provide a. What Are Catalysts In Chemistry.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 What Are Catalysts In Chemistry Catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts are conventionally divided into two categories: Catalysts allow a reaction to proceed via a pathway that has a. What Are Catalysts In Chemistry.
From news.emory.edu
Chemical Catalyst Turns 'Trash' to 'Treasure' What Are Catalysts In Chemistry This process is called catalysis. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. Catalysts provide alternative reaction pathways. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the. What Are Catalysts In Chemistry.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation ID4503154 What Are Catalysts In Chemistry Catalysts provide alternative reaction pathways. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for. What Are Catalysts In Chemistry.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of What Are Catalysts In Chemistry Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Learn how catalysts work, why they are important and what types of. Catalysts are conventionally divided into two categories: A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching. What Are Catalysts In Chemistry.
From www.nagwa.com
Lesson Catalysts Nagwa What Are Catalysts In Chemistry In heterogeneous catalysis, catalysts provide a surface to. Learn how catalysts work, why they are important and what types of. Catalysts are conventionally divided into two categories: Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Catalysts provide alternative reaction pathways. This process is called catalysis. A catalyst is another. What Are Catalysts In Chemistry.
From gamesmartz.com
Catalyst Definition & Image GameSmartz What Are Catalysts In Chemistry A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is not consumed by the reaction and it may participate in multiple reactions at a time. Catalysts provide alternative reaction pathways. Catalysts are conventionally divided into two categories: A catalyst is a material. What Are Catalysts In Chemistry.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube What Are Catalysts In Chemistry In heterogeneous catalysis, catalysts provide a surface to. A catalyst is another substance than reactants products added to a reaction system to alter the speed of a chemical reaction approaching a chemical equilibrium. A catalyst is a material that speeds up chemical reactions by lowering the activation energy. This process is called catalysis. Catalysts allow a reaction to proceed via. What Are Catalysts In Chemistry.