Iron 3 Oxide Gram . The iron (iii) oxide formula comprises iron and oxygen elements. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. the molar mass of iron (iii) oxide is 159.69 grams/mole. Convert grams iron(iii) oxide to moles. Molar mass of fe2o3 = 159.6882 g/mol. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. Iron, located in group 8 of the. iron(iii) oxide molecular weight. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. For example, water is h. Write down the chemical formula of the compound. steps to calculate molar mass. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. table of contents.
from www.numerade.com
For example, water is h. Write down the chemical formula of the compound. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. iron(iii) oxide molecular weight. Iron, located in group 8 of the. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. Molar mass of fe2o3 = 159.6882 g/mol. the molar mass of iron (iii) oxide is 159.69 grams/mole. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. table of contents.
SOLVED Iron reacts with oxygen to form iron(III) oxide according to
Iron 3 Oxide Gram iron(iii) oxide molecular weight. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. table of contents. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. Iron, located in group 8 of the. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Write down the chemical formula of the compound. steps to calculate molar mass. The iron (iii) oxide formula comprises iron and oxygen elements. Convert grams iron(iii) oxide to moles. the molar mass of iron (iii) oxide is 159.69 grams/mole. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. Molar mass of fe2o3 = 159.6882 g/mol. iron(iii) oxide molecular weight. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. For example, water is h.
From www.numerade.com
SOLVED The thermal of iron(III) hydroxide to produce Iron 3 Oxide Gram iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. Write down the chemical formula of the compound. Iron, located in group 8 of the. table of contents. The iron (iii) oxide formula comprises iron and oxygen elements. It is one of the three main oxides of. Iron 3 Oxide Gram.
From www.numerade.com
SOLVEDThe iron(III) ion content of a solution may be found by Iron 3 Oxide Gram This is found by adding the total mass of iron and oxygen in iron (iii) oxide. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. iron(iii) oxide molecular weight. For example, water is h. table of contents. Molar mass of fe2o3 = 159.6882 g/mol. the molar mass of iron (iii) oxide is 159.69. Iron 3 Oxide Gram.
From www.coursehero.com
[Solved] Iron (III) oxide reacts with carbon monoxide to produce Iron 3 Oxide Gram The iron (iii) oxide formula comprises iron and oxygen elements. Write down the chemical formula of the compound. iron(iii) oxide molecular weight. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. iron(iii) oxide or ferric oxide. Iron 3 Oxide Gram.
From germanunioncemetery.org
Iron(III) Oxide Wikipedia Iron 3 Oxide Gram Write down the chemical formula of the compound. Molar mass of fe2o3 = 159.6882 g/mol. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. steps to calculate molar mass. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. . Iron 3 Oxide Gram.
From www.inoxia.co.uk
Buy Iron (III) Oxide at Inoxia Ltd Iron 3 Oxide Gram iron(iii) oxide molecular weight. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. Convert grams iron(iii) oxide to moles. Molar mass of fe2o3 = 159.6882 g/mol. the. Iron 3 Oxide Gram.
From www.numerade.com
SOLVED Iron reacts with oxygen to form iron(III) oxide according to Iron 3 Oxide Gram the molar mass of iron (iii) oxide is 159.69 grams/mole. iron(iii) oxide molecular weight. steps to calculate molar mass. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. For example, water is h. The iron. Iron 3 Oxide Gram.
From www.chegg.com
Solved What mass (in grams) of iron(III) oxide contains 62.0 Iron 3 Oxide Gram iron(iii) oxide molecular weight. Iron, located in group 8 of the. The iron (iii) oxide formula comprises iron and oxygen elements. the molar mass of iron (iii) oxide is 159.69 grams/mole. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Write down the chemical formula of the compound. iron(iii) oxide. Iron 3 Oxide Gram.
From www.carolina.com
Iron (II, III) Oxide, Black, Powder, Laboratory Grade, 500 g Carolina Iron 3 Oxide Gram iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. Molar mass of fe2o3 = 159.6882 g/mol. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. steps to calculate molar mass. The iron (iii) oxide formula comprises iron. Iron 3 Oxide Gram.
From www.numerade.com
SOLVED The thermite reaction involves aluminum and iron(III) oxide Iron 3 Oxide Gram Convert grams iron(iii) oxide to moles. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. The iron (iii) oxide formula comprises iron and oxygen elements. For example, water is h. Iron, located in group 8 of the. . Iron 3 Oxide Gram.
From www.sciencephoto.com
Iron(III) Oxide Stock Image C030/8153 Science Photo Library Iron 3 Oxide Gram Molar mass of fe2o3 = 159.6882 g/mol. the molar mass of iron (iii) oxide is 159.69 grams/mole. Convert grams iron(iii) oxide to moles. steps to calculate molar mass. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. iron(iii) oxide or ferric oxide is the inorganic compound with the. Iron 3 Oxide Gram.
From www.researchgate.net
Mineral pigments in the iron(III) oxide/hydroxide system. Download Iron 3 Oxide Gram iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. steps to calculate molar mass. Convert grams iron(iii) oxide to moles. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. iron(iii) oxide molecular weight. The iron (iii) oxide formula comprises iron and oxygen elements. Iron, located in group. Iron 3 Oxide Gram.
From www.numerade.com
SOLVED Iron (III) oxide reacts with carbon to give iron and carbon Iron 3 Oxide Gram the molar mass of iron (iii) oxide is 159.69 grams/mole. The iron (iii) oxide formula comprises iron and oxygen elements. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. table of contents. iron(iii) oxide is chemically known as fe. Iron 3 Oxide Gram.
From www.coursehero.com
[Solved] Iron (III) oxide is formed when iron combines with oxygen in Iron 3 Oxide Gram iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. the molar mass of iron (iii) oxide is 159.69 grams/mole. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. This is found by adding the total mass of iron and oxygen in iron. Iron 3 Oxide Gram.
From www.numerade.com
SOLVEDCalculate the weight percent of iron in Fe2 O3, iron (III) oxide Iron 3 Oxide Gram iron(iii) oxide molecular weight. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. For example, water is h. Iron, located in group 8 of the. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. iron(iii) oxide reacts with sulphuric acid to produce. Iron 3 Oxide Gram.
From www.numerade.com
SOLVEDTo convert 1 mol of iron(III) oxide to its elements requires 196 Iron 3 Oxide Gram The iron (iii) oxide formula comprises iron and oxygen elements. steps to calculate molar mass. the molar mass of iron (iii) oxide is 159.69 grams/mole. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. Molar mass of fe2o3 = 159.6882 g/mol. iron(iii) oxide is chemically known as fe. Iron 3 Oxide Gram.
From www.numerade.com
SOLVED 1. Iron reacts with oxygen to produce iron(III)oxide =. The Iron 3 Oxide Gram iron(iii) oxide molecular weight. Molar mass of fe2o3 = 159.6882 g/mol. Iron, located in group 8 of the. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. steps to calculate molar. Iron 3 Oxide Gram.
From en.wikipedia.org
Iron(III) oxide Wikipedia Iron 3 Oxide Gram Write down the chemical formula of the compound. Iron, located in group 8 of the. table of contents. For example, water is h. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. Molar mass of fe2o3 = 159.6882 g/mol. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound. Iron 3 Oxide Gram.
From www.numerade.com
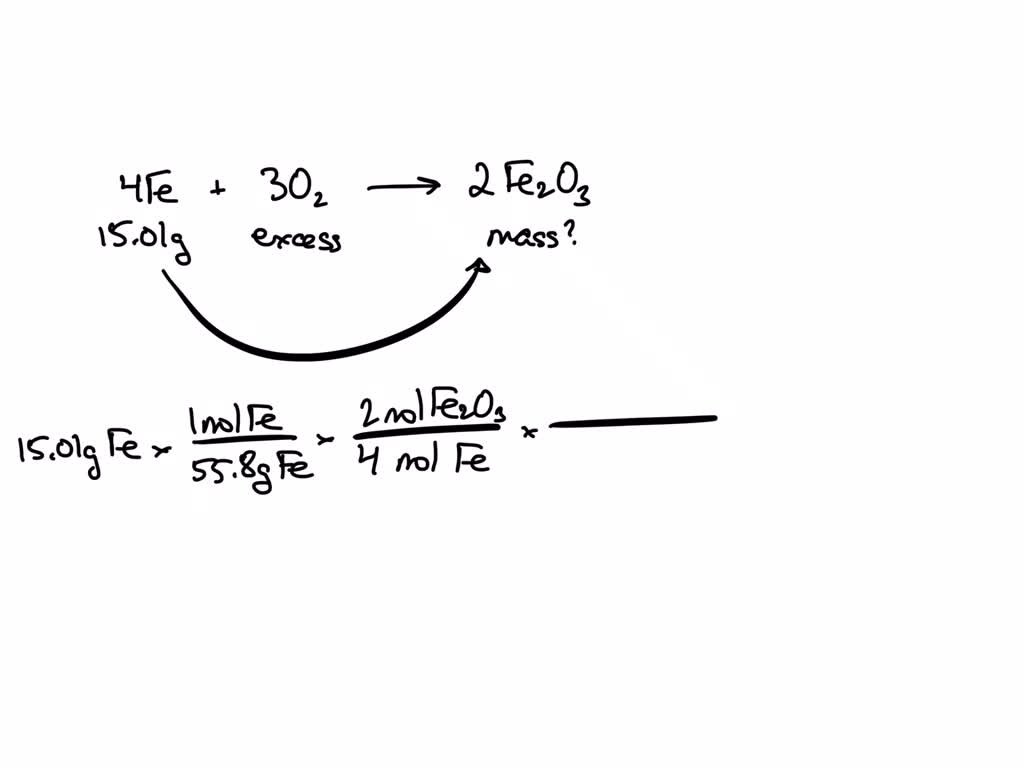
SOLVED 15.0 grams of Iron is allowed to react with air to produce Iron Iron 3 Oxide Gram steps to calculate molar mass. Write down the chemical formula of the compound. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. Convert grams iron(iii) oxide to moles. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. For example, water is h. Molar. Iron 3 Oxide Gram.
From dxoqwhxxy.blob.core.windows.net
Iron Iii Oxide Structure at Tammy Hart blog Iron 3 Oxide Gram Convert grams iron(iii) oxide to moles. For example, water is h. table of contents. steps to calculate molar mass. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. iron(iii) oxide is chemically known as fe 2. Iron 3 Oxide Gram.
From www.solutionspile.com
[Solved] Under certain conditions, the substances iron and Iron 3 Oxide Gram For example, water is h. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. the molar mass of iron (iii) oxide is 159.69 grams/mole. Iron, located in group 8 of the. iron(iii). Iron 3 Oxide Gram.
From www.youtube.com
when iron rusts, solid iron reacts with gaseous oxygen to form solid Iron 3 Oxide Gram the molar mass of iron (iii) oxide is 159.69 grams/mole. Write down the chemical formula of the compound. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. iron(iii) oxide is chemically known as fe 2 o 3,. Iron 3 Oxide Gram.
From www.youtube.com
How to Write the Formula for Iron (III) Oxide YouTube Iron 3 Oxide Gram Convert grams iron(iii) oxide to moles. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. The iron (iii) oxide formula comprises iron and oxygen elements. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. It is one of the three. Iron 3 Oxide Gram.
From www.numerade.com
SOLVEDIron metal reacts with oxygen to give iron(III) oxide, Fe2 O3 (a Iron 3 Oxide Gram For example, water is h. The iron (iii) oxide formula comprises iron and oxygen elements. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. iron(iii) oxide molecular weight. steps to calculate molar mass. iron(iii) oxide is. Iron 3 Oxide Gram.
From www.slideserve.com
PPT Stoichiometry with a Twist PowerPoint Presentation ID1836706 Iron 3 Oxide Gram the molar mass of iron (iii) oxide is 159.69 grams/mole. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. steps to calculate molar mass. Write down the chemical formula. Iron 3 Oxide Gram.
From www.numerade.com
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a Iron 3 Oxide Gram This is found by adding the total mass of iron and oxygen in iron (iii) oxide. The iron (iii) oxide formula comprises iron and oxygen elements. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. the molar mass of iron (iii) oxide is 159.69 grams/mole. Convert grams iron(iii) oxide to moles. It is one. Iron 3 Oxide Gram.
From www.youtube.com
Formation of Rust Hydrated Iron (III) Oxide YouTube Iron 3 Oxide Gram iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. table of contents. Iron, located in group 8 of the. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water.. Iron 3 Oxide Gram.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Iron 3 Oxide Gram iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. Iron, located in group 8 of the. steps to calculate molar mass. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. the molar mass of iron (iii) oxide is 159.69 grams/mole.. Iron 3 Oxide Gram.
From www.numerade.com
SOLVED The thermite reaction involves aluminum and iron(III) oxide Iron 3 Oxide Gram The iron (iii) oxide formula comprises iron and oxygen elements. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. For example, water is h. Molar mass of fe2o3 = 159.6882 g/mol. It is. Iron 3 Oxide Gram.
From dxosytfoi.blob.core.windows.net
Iron(Iii) Oxide Compound Name at Matthew Pina blog Iron 3 Oxide Gram This is found by adding the total mass of iron and oxygen in iron (iii) oxide. the molar mass of iron (iii) oxide is 159.69 grams/mole. Iron, located in group 8 of the. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. Molar mass of fe2o3 = 159.6882 g/mol. . Iron 3 Oxide Gram.
From www.numerade.com
SOLVED Determine the mass of Iron (III) oxide (Fe2O3) produced in the Iron 3 Oxide Gram Molar mass of fe2o3 = 159.6882 g/mol. This is found by adding the total mass of iron and oxygen in iron (iii) oxide. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. Convert grams iron(iii) oxide to moles. the molar mass of iron (iii) oxide is 159.69 grams/mole. iron(iii) oxide reacts with sulphuric. Iron 3 Oxide Gram.
From www.pw.live
Iron III Oxide Formula, Structure, Properties, Uses Iron 3 Oxide Gram iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. Convert grams iron(iii) oxide to moles. iron(iii) oxide molecular weight. the molar mass of iron (iii) oxide is 159.69 grams/mole. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. Molar mass of. Iron 3 Oxide Gram.
From www.chegg.com
Solved 3. Iron reacts with oxygen to form iron (III) oxide Iron 3 Oxide Gram Molar mass of fe2o3 = 159.6882 g/mol. Convert grams iron(iii) oxide to moles. iron(iii) oxide molecular weight. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. table of contents. the molar mass of iron (iii) oxide is 159.69 grams/mole. steps to calculate molar. Iron 3 Oxide Gram.
From www.youtube.com
How to write the formula for iron (III) oxide YouTube Iron 3 Oxide Gram iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. The iron (iii) oxide formula comprises iron and oxygen elements. table of contents. Molar mass of fe2o3 = 159.6882 g/mol. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. the molar mass of iron (iii) oxide is 159.69 grams/mole. Iron,. Iron 3 Oxide Gram.
From www.numerade.com
SOLVED Calculate the mass of Iron(III) oxide (FezO;) that contains a Iron 3 Oxide Gram For example, water is h. Write down the chemical formula of the compound. steps to calculate molar mass. iron(iii) oxide or ferric oxide is the inorganic compound with the formula fe2o3. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. table of contents. Iron, located in group 8 of the. The iron (iii). Iron 3 Oxide Gram.
From www.numerade.com
SOLVED For the following reaction, 8 5 . 0 grams of iron are allowed Iron 3 Oxide Gram steps to calculate molar mass. iron(iii) oxide reacts with sulphuric acid to produce iron(iii) sulfate and water. iron(iii) oxide is chemically known as fe 2 o 3, which is an inorganic compound also referred to as hematite or. It is one of the three main oxides of iron, the other two being iron(ii) oxide (feo) the. This. Iron 3 Oxide Gram.