Can You Have Red Indicators . these natural ph indicators include: indicators are substances whose solutions change color due to changes in ph. If the indicator is a weak acid, the acid and its conjugate base are different colors. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. in solutions where ph > p ka, the logarithmic term must be positive, indicating an excess of the conjugate base form of the indicator. The undissociated form of the indicator is a different color than the iogenic form of the indicator. updated on november 04, 2019. A very basic solution (high ph) will change the color of beets or beet juice from red to purple. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. Blackberries, black currants, and black raspberries change from red in an acidic environment to blue or violet in a basic environment. They are usually weak acids or.
from thechemistrynotes.com
A very basic solution (high ph) will change the color of beets or beet juice from red to purple. Blackberries, black currants, and black raspberries change from red in an acidic environment to blue or violet in a basic environment. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. The undissociated form of the indicator is a different color than the iogenic form of the indicator. updated on november 04, 2019. indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or. in solutions where ph > p ka, the logarithmic term must be positive, indicating an excess of the conjugate base form of the indicator. If the indicator is a weak acid, the acid and its conjugate base are different colors.
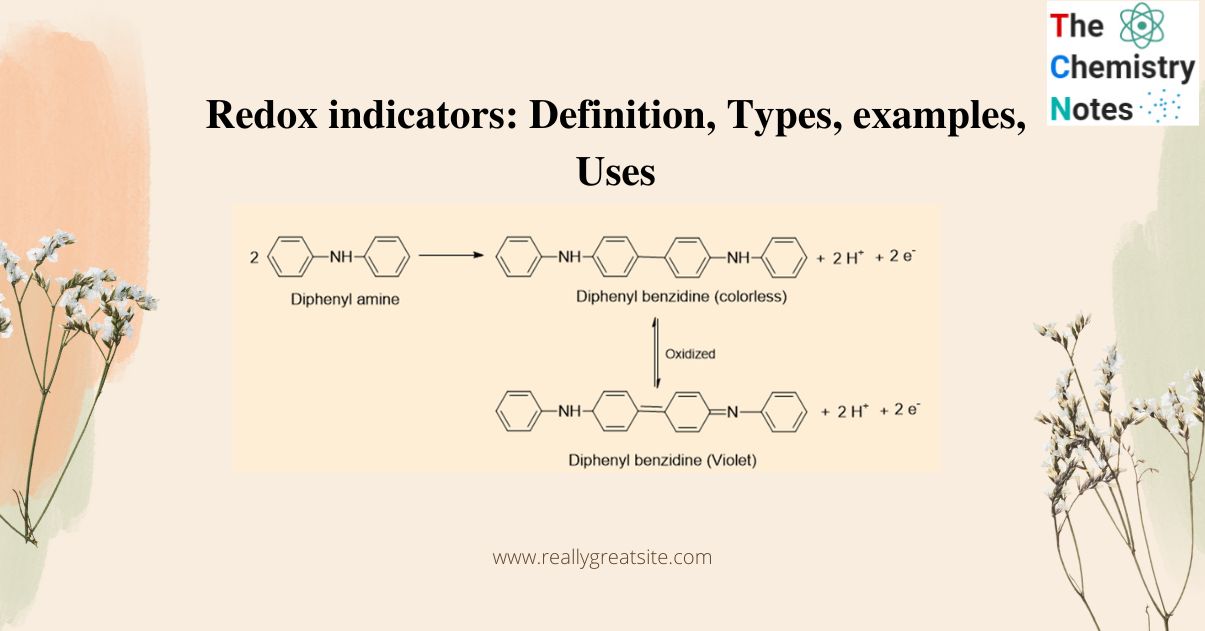
Redox indicators
Can You Have Red Indicators A very basic solution (high ph) will change the color of beets or beet juice from red to purple. They are usually weak acids or. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. these natural ph indicators include: If the indicator is a weak acid, the acid and its conjugate base are different colors. in solutions where ph > p ka, the logarithmic term must be positive, indicating an excess of the conjugate base form of the indicator. A very basic solution (high ph) will change the color of beets or beet juice from red to purple. Blackberries, black currants, and black raspberries change from red in an acidic environment to blue or violet in a basic environment. The undissociated form of the indicator is a different color than the iogenic form of the indicator. updated on november 04, 2019. indicators are substances whose solutions change color due to changes in ph. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or.
From www.ctlabs.ca
Executive Dashboarditis or "does that red light actually work???" CTLabs Can You Have Red Indicators in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. They are usually weak acids or. these natural ph indicators include: A very basic solution (high ph) will change the color of beets or beet juice from red to purple. Blackberries, black currants, and. Can You Have Red Indicators.
From www.choppershop.com
Light Red Indicator Can You Have Red Indicators as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. The undissociated form of the indicator is a different color than the iogenic form of the indicator. these natural ph indicators include: If the indicator is a weak acid, the acid and its conjugate base are. Can You Have Red Indicators.
From pngtree.com
Indicator Clipart Hd PNG, Red Indicator, Red, Indicative Mark, Label PNG Image For Free Download Can You Have Red Indicators updated on november 04, 2019. these natural ph indicators include: Blackberries, black currants, and black raspberries change from red in an acidic environment to blue or violet in a basic environment. If the indicator is a weak acid, the acid and its conjugate base are different colors. in more basic solutions where the hydronium ion concentration is. Can You Have Red Indicators.
From can.grandado.com
10mm red indicator light 10mm pilot light 220V 24V indicator light 220V 110V 12V/24V Panel Can You Have Red Indicators If the indicator is a weak acid, the acid and its conjugate base are different colors. in solutions where ph > p ka, the logarithmic term must be positive, indicating an excess of the conjugate base form of the indicator. They are usually weak acids or. The undissociated form of the indicator is a different color than the iogenic. Can You Have Red Indicators.
From www.walmart.com
Seachoice LED Red Indicator Light Can You Have Red Indicators these natural ph indicators include: in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. If the indicator is a weak acid, the acid and its conjugate base are different colors. They are usually weak acids or. updated on november 04, 2019. The. Can You Have Red Indicators.
From www.hnhcart.com
Buy Online 220V AC Red Indicator Led Light in India Hnhcart Can You Have Red Indicators updated on november 04, 2019. They are usually weak acids or. these natural ph indicators include: The undissociated form of the indicator is a different color than the iogenic form of the indicator. A very basic solution (high ph) will change the color of beets or beet juice from red to purple. in more basic solutions where. Can You Have Red Indicators.
From pngtree.com
Red Indicator Clipart PNG, Vector, PSD, and Clipart With Transparent Background for Free Can You Have Red Indicators They are usually weak acids or. A very basic solution (high ph) will change the color of beets or beet juice from red to purple. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. The undissociated form of the indicator is a different color than the. Can You Have Red Indicators.
From www.alamy.com
the red indicator light blinks Stock Video Footage Alamy Can You Have Red Indicators They are usually weak acids or. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. updated on november 04, 2019. these natural ph indicators include: in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph. Can You Have Red Indicators.
From connect2india.com
Red Indicator Light Suppliers Red Indicator Light विक्रेता and आपूर्तिकर्ता Suppliers of Red Can You Have Red Indicators They are usually weak acids or. updated on november 04, 2019. in solutions where ph > p ka, the logarithmic term must be positive, indicating an excess of the conjugate base form of the indicator. A very basic solution (high ph) will change the color of beets or beet juice from red to purple. as you go. Can You Have Red Indicators.
From www.compoundchem.com
Making a Red Cabbage pH Indicator The Method and the Chemistry Compound Interest Can You Have Red Indicators indicators are substances whose solutions change color due to changes in ph. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red. Can You Have Red Indicators.
From dxohctahh.blob.core.windows.net
Examples Of Indicators at Steve Allen blog Can You Have Red Indicators in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. these natural ph indicators include: They are usually weak acids or. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow.. Can You Have Red Indicators.
From heartautocare.com
Car Warning Lights HEART Certified Auto Care Can You Have Red Indicators Blackberries, black currants, and black raspberries change from red in an acidic environment to blue or violet in a basic environment. The undissociated form of the indicator is a different color than the iogenic form of the indicator. If the indicator is a weak acid, the acid and its conjugate base are different colors. these natural ph indicators include:. Can You Have Red Indicators.
From exorrglvg.blob.core.windows.net
Synthetic Indicators Examples Class 7 at Andrew Cardenas blog Can You Have Red Indicators They are usually weak acids or. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. If the indicator is a weak acid, the acid and its conjugate base are different colors. in more basic solutions where the hydronium ion concentration is less than 5.0 ×. Can You Have Red Indicators.
From www.vectorstock.com
Ph scale universal indicator color chart Vector Image Can You Have Red Indicators If the indicator is a weak acid, the acid and its conjugate base are different colors. They are usually weak acids or. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. as you go on adding more acid, the red will eventually become. Can You Have Red Indicators.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Can You Have Red Indicators A very basic solution (high ph) will change the color of beets or beet juice from red to purple. updated on november 04, 2019. indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or. The undissociated form of the indicator is a different color than the iogenic form of the. Can You Have Red Indicators.
From dxofrcamx.blob.core.windows.net
What Is The Meaning Of Indicator In Chemistry at Laura Nelson blog Can You Have Red Indicators A very basic solution (high ph) will change the color of beets or beet juice from red to purple. updated on november 04, 2019. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. The undissociated form of the indicator is a different color than the. Can You Have Red Indicators.
From www.science-sparks.com
How to make a red cabbage pH indicator Chemistry for Kids Can You Have Red Indicators updated on november 04, 2019. A very basic solution (high ph) will change the color of beets or beet juice from red to purple. in solutions where ph > p ka, the logarithmic term must be positive, indicating an excess of the conjugate base form of the indicator. The undissociated form of the indicator is a different color. Can You Have Red Indicators.
From www.johnnylawmotors.com
Red Indicator with Chrome Ring Can You Have Red Indicators The undissociated form of the indicator is a different color than the iogenic form of the indicator. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. these natural ph indicators include: updated on november 04, 2019. A very basic solution (high ph) will change. Can You Have Red Indicators.
From sciencenotes.org
Universal Indicator Chart and Recipes Can You Have Red Indicators The undissociated form of the indicator is a different color than the iogenic form of the indicator. A very basic solution (high ph) will change the color of beets or beet juice from red to purple. updated on november 04, 2019. as you go on adding more acid, the red will eventually become so dominant that you can. Can You Have Red Indicators.
From exohhvfwy.blob.core.windows.net
What Does An Indicator Help Us See at Deloris Richards blog Can You Have Red Indicators in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. They are usually weak acids or. Blackberries, black currants, and black raspberries change from red in an acidic environment to blue or violet in a basic environment. as you go on adding more acid,. Can You Have Red Indicators.
From www.rhythmsystems.com
Setting Red Yellow Green Goal Performance Indicator Examples That Are SMART Can You Have Red Indicators If the indicator is a weak acid, the acid and its conjugate base are different colors. updated on november 04, 2019. Blackberries, black currants, and black raspberries change from red in an acidic environment to blue or violet in a basic environment. They are usually weak acids or. The undissociated form of the indicator is a different color than. Can You Have Red Indicators.
From udvabony.com
RED Indicator Light AC 250V 5A Heavy Duty RED Indicator Can You Have Red Indicators If the indicator is a weak acid, the acid and its conjugate base are different colors. updated on november 04, 2019. A very basic solution (high ph) will change the color of beets or beet juice from red to purple. They are usually weak acids or. Blackberries, black currants, and black raspberries change from red in an acidic environment. Can You Have Red Indicators.
From www.alamy.com
Vertical red indicator with arrow pointer. Thinning and thickening, measurement dashboard, arrow Can You Have Red Indicators as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. The undissociated form of the indicator is a different color than the iogenic form of the indicator. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph >. Can You Have Red Indicators.
From www.dreamstime.com
Red Indicator User Interface with Smile Face Stock Vector Illustration of indicator, metering Can You Have Red Indicators these natural ph indicators include: The undissociated form of the indicator is a different color than the iogenic form of the indicator. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. in solutions where ph > p ka, the logarithmic term must. Can You Have Red Indicators.
From www.slideserve.com
PPT Litmus Indicator PowerPoint Presentation, free download ID2790323 Can You Have Red Indicators A very basic solution (high ph) will change the color of beets or beet juice from red to purple. Blackberries, black currants, and black raspberries change from red in an acidic environment to blue or violet in a basic environment. in solutions where ph > p ka, the logarithmic term must be positive, indicating an excess of the conjugate. Can You Have Red Indicators.
From led-indicator.com
19mm red led ip67 metal pilot indicator light with a wireLed Indicator Can You Have Red Indicators updated on november 04, 2019. in solutions where ph > p ka, the logarithmic term must be positive, indicating an excess of the conjugate base form of the indicator. Blackberries, black currants, and black raspberries change from red in an acidic environment to blue or violet in a basic environment. A very basic solution (high ph) will change. Can You Have Red Indicators.
From www.ibuychemikals.com
Neutral Red Indicator AR buy online at ibuychemikals CAS No.553242 Can You Have Red Indicators The undissociated form of the indicator is a different color than the iogenic form of the indicator. A very basic solution (high ph) will change the color of beets or beet juice from red to purple. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. Blackberries,. Can You Have Red Indicators.
From alertelectrical.com
Knightsbridge Curved Red indicator module. CUGM13 Can You Have Red Indicators updated on november 04, 2019. indicators are substances whose solutions change color due to changes in ph. these natural ph indicators include: A very basic solution (high ph) will change the color of beets or beet juice from red to purple. If the indicator is a weak acid, the acid and its conjugate base are different colors.. Can You Have Red Indicators.
From www.science-sparks.com
How to make a red cabbage pH indicator Chemistry for Kids Can You Have Red Indicators in solutions where ph > p ka, the logarithmic term must be positive, indicating an excess of the conjugate base form of the indicator. The undissociated form of the indicator is a different color than the iogenic form of the indicator. these natural ph indicators include: in more basic solutions where the hydronium ion concentration is less. Can You Have Red Indicators.
From thechemistrynotes.com
Redox indicators Can You Have Red Indicators A very basic solution (high ph) will change the color of beets or beet juice from red to purple. as you go on adding more acid, the red will eventually become so dominant that you can no longer see any yellow. They are usually weak acids or. in more basic solutions where the hydronium ion concentration is less. Can You Have Red Indicators.
From www.vectorstock.com
Vertical red indicator with arrow pointer Vector Image Can You Have Red Indicators The undissociated form of the indicator is a different color than the iogenic form of the indicator. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. If the indicator is a weak acid, the acid and its conjugate base are different colors. Blackberries, black. Can You Have Red Indicators.
From hubpages.com
What is Universal Indicator and How To Use it Can You Have Red Indicators in solutions where ph > p ka, the logarithmic term must be positive, indicating an excess of the conjugate base form of the indicator. indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or. as you go on adding more acid, the red will eventually become so dominant that. Can You Have Red Indicators.
From www.indiamart.com
7021 Red Indicator With LED at Rs 169/piece Indicator Lamp in Thane ID 17687398448 Can You Have Red Indicators in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. They are usually weak acids or. If the indicator is a weak acid, the acid and its conjugate base are different colors. A very basic solution (high ph) will change the color of beets or. Can You Have Red Indicators.
From www.youtube.com
a Red indicator light on a dashboard indicates YouTube Can You Have Red Indicators updated on november 04, 2019. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. Blackberries, black currants, and black raspberries change from red in an acidic environment to blue or violet in a basic environment. A very basic solution (high ph) will change. Can You Have Red Indicators.
From www.walmart.com
Uxcell 10mm Thread 220V Red LED Light Indicator Signal Pilot Lamp Can You Have Red Indicators indicators are substances whose solutions change color due to changes in ph. in more basic solutions where the hydronium ion concentration is less than 5.0 × 10 −9 m (ph > 8.3), it is red or. Blackberries, black currants, and black raspberries change from red in an acidic environment to blue or violet in a basic environment. . Can You Have Red Indicators.