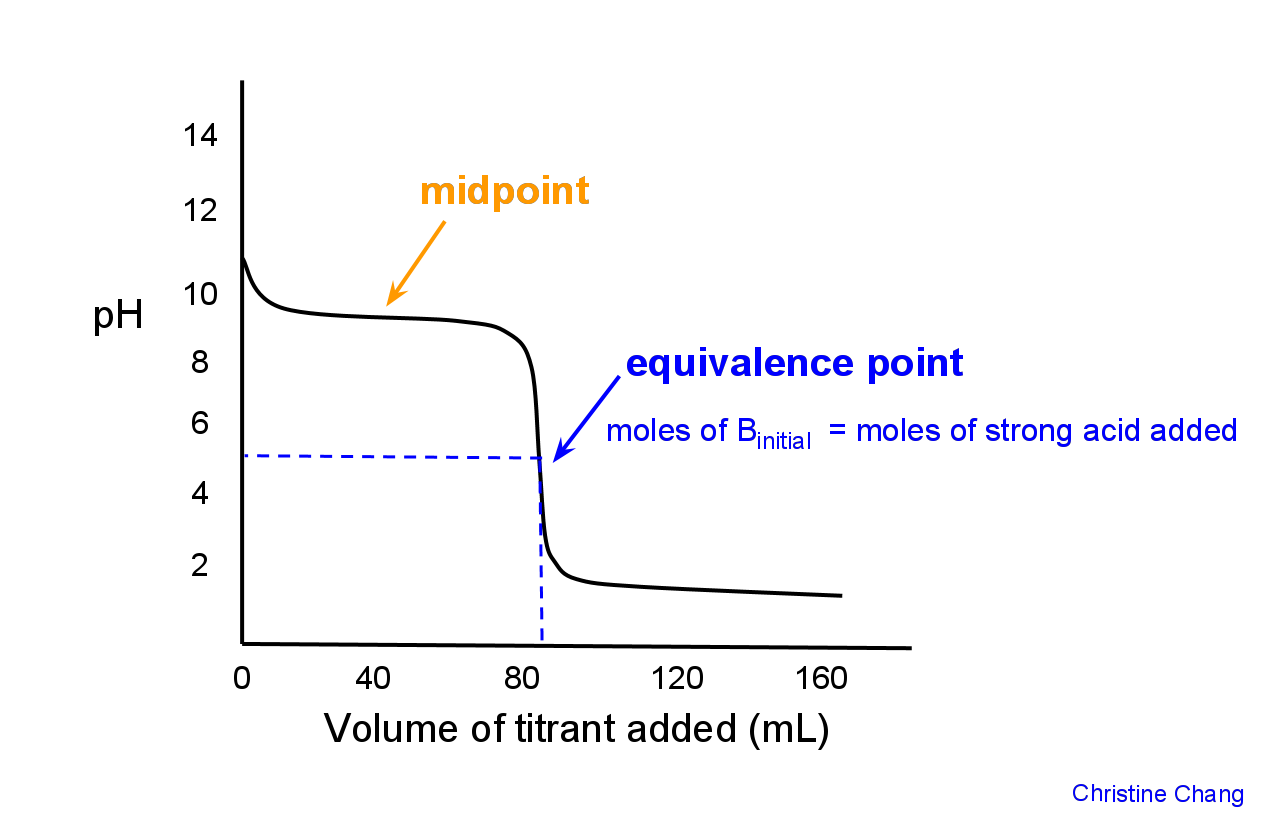
Strong Base Titration Equivalence Point . past the equivalence point. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. postequivalence point (v > 25 ml): equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. The equivalence point of a titration. Ph is determined by the amount of excess strong base titrant added; the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an.
from chem.libretexts.org
Ph is determined by the amount of excess strong base titrant added; the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. past the equivalence point. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. The equivalence point of a titration. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. postequivalence point (v > 25 ml): Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is.
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts
Strong Base Titration Equivalence Point The equivalence point of a titration. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. past the equivalence point. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. Ph is determined by the amount of excess strong base titrant added; postequivalence point (v > 25 ml): The equivalence point of a titration. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Strong Base Titration Equivalence Point past the equivalence point. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. postequivalence point (v > 25 ml): for the titration of a strong acid with a. Strong Base Titration Equivalence Point.
From www.youtube.com
Titration Weak Acid Strong Base Equivalence Point YouTube Strong Base Titration Equivalence Point Ph is determined by the amount of excess strong base titrant added; Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. postequivalence point (v > 25 ml): the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide. Strong Base Titration Equivalence Point.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Strong Base Titration Equivalence Point Ph is determined by the amount of excess strong base titrant added; for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid). Strong Base Titration Equivalence Point.
From www.chm.uri.edu
CHM 112 Lecture 19 Strong Base Titration Equivalence Point The equivalence point of a titration. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. past the equivalence. Strong Base Titration Equivalence Point.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Strong Base Titration Equivalence Point the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. . Strong Base Titration Equivalence Point.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID5369229 Strong Base Titration Equivalence Point postequivalence point (v > 25 ml): for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. The equivalence point of a titration. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or. Strong Base Titration Equivalence Point.
From schoolbag.info
Titration and Buffers Acids and Bases Strong Base Titration Equivalence Point postequivalence point (v > 25 ml): The equivalence point of a titration. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. past the equivalence point.. Strong Base Titration Equivalence Point.
From www.youtube.com
Weak acid / strong base titration pH after equivalence point YouTube Strong Base Titration Equivalence Point for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. the titration curve for the titration of 25.00 ml. Strong Base Titration Equivalence Point.
From mungfali.com
Titration Curve Labeled Strong Base Titration Equivalence Point Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. past the equivalence point. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. Ph is determined by the amount of excess strong base titrant added; The equivalence point of a. Strong Base Titration Equivalence Point.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Strong Base Titration Equivalence Point Ph is determined by the amount of excess strong base titrant added; The equivalence point of a titration. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. postequivalence point (v. Strong Base Titration Equivalence Point.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Strong Base Titration Equivalence Point past the equivalence point. Ph is determined by the amount of excess strong base titrant added; equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. postequivalence point (v > 25 ml): for the titration of a strong acid with a strong base, the equivalence point. Strong Base Titration Equivalence Point.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Strong Base Titration Equivalence Point Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. postequivalence point (v > 25 ml): the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a strong acid with a strong. Strong Base Titration Equivalence Point.
From dxomvqiez.blob.core.windows.net
Equivalence Point For Titration Of Strong Acid at Ora Peach blog Strong Base Titration Equivalence Point for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. past the equivalence point. equivalence (stoichiometric, s) point. Strong Base Titration Equivalence Point.
From dokumen.tips
(PPT) TITRATION Titration of a strong acid with a strong base ENDPOINT = POINT OF NEUTRALIZATION Strong Base Titration Equivalence Point postequivalence point (v > 25 ml): for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. past the equivalence point. the titration of a weak acid with a strong base involves the direct transfer. Strong Base Titration Equivalence Point.
From www.slideserve.com
PPT AcidBase Titration PowerPoint Presentation, free download ID5369229 Strong Base Titration Equivalence Point Ph is determined by the amount of excess strong base titrant added; the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. The equivalence point of a titration. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles,. Strong Base Titration Equivalence Point.
From www.youtube.com
Titration Weak base/Strong acid Equivalence Point YouTube Strong Base Titration Equivalence Point Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. The equivalence point of a titration. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh. Strong Base Titration Equivalence Point.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Strong Base Titration Equivalence Point the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. Ph is determined by the amount of excess strong base titrant added; for. Strong Base Titration Equivalence Point.
From www.numerade.com
SOLVEDDraw the general titration curve for a strong acid titrated with a strong base. At the Strong Base Titration Equivalence Point Ph is determined by the amount of excess strong base titrant added; postequivalence point (v > 25 ml): for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. past the equivalence point. the titration. Strong Base Titration Equivalence Point.
From www.youtube.com
Weak acid / strong base titration pH at equivalence point YouTube Strong Base Titration Equivalence Point Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. The equivalence point of a titration. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. the titration of a weak acid with a strong base. Strong Base Titration Equivalence Point.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Chemistry JoVe Strong Base Titration Equivalence Point the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. Ph is. Strong Base Titration Equivalence Point.
From exolxuobk.blob.core.windows.net
How To Do Strong Acid Strong Base Titration at Cole blog Strong Base Titration Equivalence Point The equivalence point of a titration. equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution.. Strong Base Titration Equivalence Point.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Strong Base Titration Equivalence Point the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. postequivalence point (v > 25 ml): past the equivalence point. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the. Strong Base Titration Equivalence Point.
From www.slideshare.net
pH Understanding titration curve Strong Base Titration Equivalence Point the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. The equivalence point of a titration. postequivalence point (v > 25 ml): past the equivalence point. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak. Strong Base Titration Equivalence Point.
From dxomvqiez.blob.core.windows.net
Equivalence Point For Titration Of Strong Acid at Ora Peach blog Strong Base Titration Equivalence Point postequivalence point (v > 25 ml): for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. The equivalence point of a titration. the titration of a weak acid with a strong base involves the direct. Strong Base Titration Equivalence Point.
From philschatz.com
AcidBase Titrations · Chemistry Strong Base Titration Equivalence Point Ph is determined by the amount of excess strong base titrant added; the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the. Strong Base Titration Equivalence Point.
From www.youtube.com
Finding the pH during titration of a weak acid with a strong base, at the equivalence point Strong Base Titration Equivalence Point the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. past the equivalence point. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be. Strong Base Titration Equivalence Point.
From vdocuments.mx
Titration of a weak acid with a strong base pH calculations 1. Before base addition 2. From Strong Base Titration Equivalence Point equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and. Strong Base Titration Equivalence Point.
From courses.lumenlearning.com
14.8 AcidBase Titrations General College Chemistry II Strong Base Titration Equivalence Point postequivalence point (v > 25 ml): equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. past the equivalence point. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. Ph is determined by the amount of excess strong base. Strong Base Titration Equivalence Point.
From www.youtube.com
Strong AcidStrong Base titration, points 1 and 2 YouTube Strong Base Titration Equivalence Point Ph is determined by the amount of excess strong base titrant added; the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong. Strong Base Titration Equivalence Point.
From www.youtube.com
Determining the pH During a Strong AcidWeak Base Titration at Equivalence YouTube Strong Base Titration Equivalence Point Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. The equivalence point of a titration. past the equivalence point. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a strong. Strong Base Titration Equivalence Point.
From slideplayer.com
Titration A pH meter or indicators are used to determine when the solution has reached the Strong Base Titration Equivalence Point postequivalence point (v > 25 ml): The equivalence point of a titration. the titration curve for the titration of 25.00 ml of 0.100 m ch 3 cooh (weak acid) with 0.100 m naoh (strong base) has an. Ph is determined by the amount of excess strong base titrant added; equivalence (stoichiometric, s) point = theoretical volume at. Strong Base Titration Equivalence Point.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Strong Base Titration Equivalence Point Ph is determined by the amount of excess strong base titrant added; The equivalence point of a titration. past the equivalence point. Suppose 100 ml of the 6 m strong acid titrant, which comes out to 0.6 moles, is. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak. Strong Base Titration Equivalence Point.
From www.slideserve.com
PPT TITRATION CURVE WEAK ACID WITH STRONG BASE MGKP 2014 PowerPoint Presentation ID2516572 Strong Base Titration Equivalence Point postequivalence point (v > 25 ml): Ph is determined by the amount of excess strong base titrant added; for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of 7.00 and the points on the titration curve can be calculated using solution. The equivalence point of a titration. the. Strong Base Titration Equivalence Point.
From exolxuobk.blob.core.windows.net
How To Do Strong Acid Strong Base Titration at Cole blog Strong Base Titration Equivalence Point postequivalence point (v > 25 ml): equivalence (stoichiometric, s) point = theoretical volume at which moles of base (or acid) added equals moles of acid (or. Ph is determined by the amount of excess strong base titrant added; for the titration of a strong acid with a strong base, the equivalence point occurs at a ph of. Strong Base Titration Equivalence Point.
From www.numerade.com
SOLVED Using the following pH curve for the titration of a weak acid with a strong base, if the Strong Base Titration Equivalence Point past the equivalence point. postequivalence point (v > 25 ml): The equivalence point of a titration. the titration of a weak acid with a strong base involves the direct transfer of protons from the weak acid to the hydoxide ion. for the titration of a strong acid with a strong base, the equivalence point occurs at. Strong Base Titration Equivalence Point.