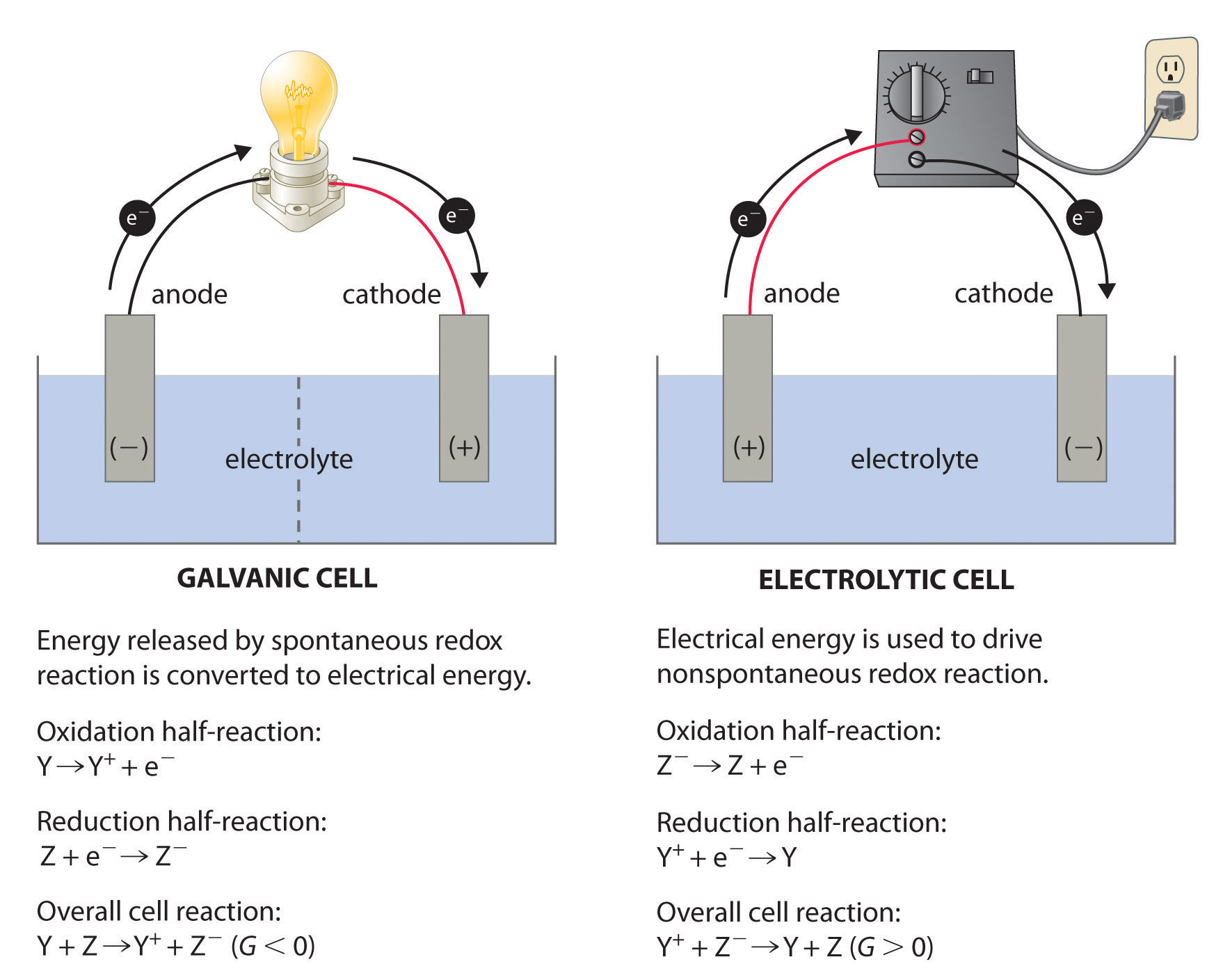
A Galvanic Battery . One example of a galvanic cell is the common household battery. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. A galvanic cell consists of at least two half. The electrons flow from one chemical reaction to. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. To understand this operation in detail, we must first understand what a redox reaction is. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means.
from chem.libretexts.org
One example of a galvanic cell is the common household battery. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. A galvanic cell consists of at least two half. To understand this operation in detail, we must first understand what a redox reaction is. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. The electrons flow from one chemical reaction to.
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts
A Galvanic Battery It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. The electrons flow from one chemical reaction to. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. To understand this operation in detail, we must first understand what a redox reaction is. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. One example of a galvanic cell is the common household battery. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. A galvanic cell consists of at least two half.
From www.geeksforgeeks.org
Electrochemical Cell Definition, Types, Working Principle & Uses A Galvanic Battery It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. A battery is a package of one or more galvanic cells used for. A Galvanic Battery.
From saylordotorg.github.io
Commercial Galvanic Cells A Galvanic Battery To understand this operation in detail, we must first understand what a redox reaction is. The electrons flow from one chemical reaction to. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. A. A Galvanic Battery.
From www.giga.de
Wie funktioniert eine Batterie? Das Prinzip der galvanischen Zellen A Galvanic Battery The electrons flow from one chemical reaction to. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. A battery is a package of one or more galvanic cells used for the production and storage of electric. A Galvanic Battery.
From courses.lumenlearning.com
Galvanic Cells General Chemistry A Galvanic Battery A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. The electrons flow from one chemical reaction to. It achieves this by harnessing the energy produced by the redox reactions that. A Galvanic Battery.
From www.researchgate.net
Schematic illustration of (a) the galvanic battery reaction and (b) ORR A Galvanic Battery It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. The electrons flow from one chemical reaction to. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. One example of a galvanic cell is the common household battery. To understand this operation in detail, we must. A Galvanic Battery.
From www.cour-electrique.com
on video How a Battery Works⚡ Galvanic Cell ⚡ REDOX Reaction Cour A Galvanic Battery A galvanic cell consists of at least two half. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. It achieves this by harnessing the energy produced by the redox reactions. A Galvanic Battery.
From dannymeta.com
Who invented a galvanic battery that used a carbon element A Galvanic Battery It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. A galvanic cell consists of at least two half. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. Sometimes. A Galvanic Battery.
From www.corrosionpedia.com
How Galvanic Corrosion Can Be Used for Good A Galvanic Battery The electrons flow from one chemical reaction to. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means.. A Galvanic Battery.
From en.wikipedia.org
Penny battery Wikipedia A Galvanic Battery One example of a galvanic cell is the common household battery. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. To understand this operation in detail, we must first understand what a redox reaction is. A battery is a package of one or more galvanic cells used for the production and storage of. A Galvanic Battery.
From www.goodreads.com
How to Use a Galvanic Battery in Medicine and Surgery by Herbert A Galvanic Battery Sometimes known as a voltaic cell or daniell cell is a galvanic cell. A galvanic cell consists of at least two half. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. A battery is a package. A Galvanic Battery.
From www.alamy.com
Galvanic Battery, that is used to produce electricity, by the A Galvanic Battery Sometimes known as a voltaic cell or daniell cell is a galvanic cell. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. A galvanic cell consists of at least two half. One example of a. A Galvanic Battery.
From www.researchgate.net
From top, (a) Ørsted's first galvanic battery using Ag and Zn A Galvanic Battery A galvanic cell consists of at least two half. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. It achieves this by harnessing the energy produced by the redox. A Galvanic Battery.
From chem.libretexts.org
Chapter 19.1 Describing Electrochemical Cells Chemistry LibreTexts A Galvanic Battery A galvanic cell consists of at least two half. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. One example of a galvanic cell is the common household battery. The electrons flow from one chemical reaction to. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical. A Galvanic Battery.
From www.alamy.com
LA2 NSRW 1 0227 Simple Galvanic Battery Stock Photo Alamy A Galvanic Battery One example of a galvanic cell is the common household battery. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. A galvanic cell consists of at least two half. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. The. A Galvanic Battery.
From www.wisegeek.com
What is the Galvanic Cell? (with picture) A Galvanic Battery One example of a galvanic cell is the common household battery. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. The electrons. A Galvanic Battery.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working A Galvanic Battery It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. One example of a galvanic cell is the common household battery. To understand this operation in detail, we must first understand what a redox reaction is. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful.. A Galvanic Battery.
From www.dreamstime.com
Battery current stock illustration. Illustration of physics 212689705 A Galvanic Battery It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. A galvanic or. A Galvanic Battery.
From chem.libretexts.org
Commercial Galvanic Cells Chemistry LibreTexts A Galvanic Battery A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. The electrons flow from one chemical reaction to. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. One example of a galvanic cell is the common household battery. A galvanic or voltaic cell. A Galvanic Battery.
From www.istockphoto.com
Galvanic Battery Stock Illustration Download Image Now 19th Century A Galvanic Battery It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. The electrons flow from one chemical reaction to. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. To understand this operation in detail, we must first understand what a redox reaction is. One example of a galvanic cell is. A Galvanic Battery.
From dokumen.tips
(PPT) Batteries A galvanic cell or a group of galvanic cells connected A Galvanic Battery Sometimes known as a voltaic cell or daniell cell is a galvanic cell. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. The electrons flow from one chemical reaction to. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. A galvanic cell consists of at. A Galvanic Battery.
From www.pinterest.com
Galvani's voltaic pile A Galvanic Battery To understand this operation in detail, we must first understand what a redox reaction is. A galvanic cell consists of at least two half. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. A battery is a package of one or more galvanic cells used for the production and storage of electric. A Galvanic Battery.
From socratic.org
How can a galvanic cell an electrolytic cell? Socratic A Galvanic Battery It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. To understand this operation in detail, we must first understand what a redox reaction is. A galvanic or voltaic cell is an electrochemical cell that converts. A Galvanic Battery.
From www.youtube.com
Electrochemistry Galvanic/Voltaic Cell Battery Made Super Simple! MCAT A Galvanic Battery To understand this operation in detail, we must first understand what a redox reaction is. One example of a galvanic cell is the common household battery. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. It is shown that, for. A Galvanic Battery.
From saylordotorg.github.io
Commercial Galvanic Cells A Galvanic Battery A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. To understand this operation in detail, we must first understand what a redox reaction is. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. It achieves this by harnessing the. A Galvanic Battery.
From favpng.com
Voltaic Pile Galvanic Cell Electric Battery Electricity Invention, PNG A Galvanic Battery A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. To understand this operation in detail, we must first understand what a redox reaction is. A galvanic cell consists of at. A Galvanic Battery.
From www.dreamstime.com
Galvanic cell or battery stock photo. Image of power 299411636 A Galvanic Battery The electrons flow from one chemical reaction to. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. One example of a galvanic cell is the common household battery. To understand this operation in detail, we must first understand what a redox reaction is. A galvanic cell consists of at least two half.. A Galvanic Battery.
From flatworldknowledge.lardbucket.org
Commercial Galvanic Cells A Galvanic Battery A galvanic cell consists of at least two half. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. It achieves this by harnessing the energy produced by the redox. A Galvanic Battery.
From sbarrchapter10-11.blogspot.com
Imperialism Galvanic Batteries A Galvanic Battery The electrons flow from one chemical reaction to. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. One example of a galvanic cell is the common household battery. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. A galvanic cell consists of at least two half. To understand. A Galvanic Battery.
From etc.usf.edu
Galvanic Battery ClipArt ETC A Galvanic Battery A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. One example of a galvanic cell is the common household. A Galvanic Battery.
From www.electroniclinic.com
What is battery? Types of battery, Primary and Secondary cells A Galvanic Battery The electrons flow from one chemical reaction to. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. Sometimes known as a voltaic cell or daniell cell is a galvanic cell. One example of a galvanic cell is the common household battery. To understand this operation in detail, we must first understand what. A Galvanic Battery.
From www.alamy.com
Engraving depicting a galvanic battery Z = zinc, C = copper Stock A Galvanic Battery To understand this operation in detail, we must first understand what a redox reaction is. A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. Sometimes known as a voltaic cell. A Galvanic Battery.
From www.peoi.org
Chapter 14 Section C Applications of Redox Reactions Voltaic Cells A Galvanic Battery A galvanic cell consists of at least two half. To understand this operation in detail, we must first understand what a redox reaction is. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. One example of a galvanic cell is the common household battery. The electrons flow from one chemical reaction to. A. A Galvanic Battery.
From www.pinterest.ph
Table of ContentsWhat is a battery? Understanding galvanic cells A Galvanic Battery A battery is a package of one or more galvanic cells used for the production and storage of electric energy by chemical means. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. The electrons flow from one chemical reaction to. Sometimes known as a voltaic cell or daniell cell is a galvanic cell.. A Galvanic Battery.
From www.alamy.com
Concept of galvanic battery Stock Photo Alamy A Galvanic Battery It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. The electrons flow from one chemical reaction to. A galvanic cell consists of at least two half. A galvanic or voltaic cell is an electrochemical cell that converts chemical energy into electrical energy. To understand this operation in detail, we must first understand. A Galvanic Battery.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure A Galvanic Battery One example of a galvanic cell is the common household battery. To understand this operation in detail, we must first understand what a redox reaction is. It is shown that, for simple galvanic cells or batteries with reactive metal electrodes, two intuitively meaningful. It achieves this by harnessing the energy produced by the redox reactions that occur within the cell.. A Galvanic Battery.