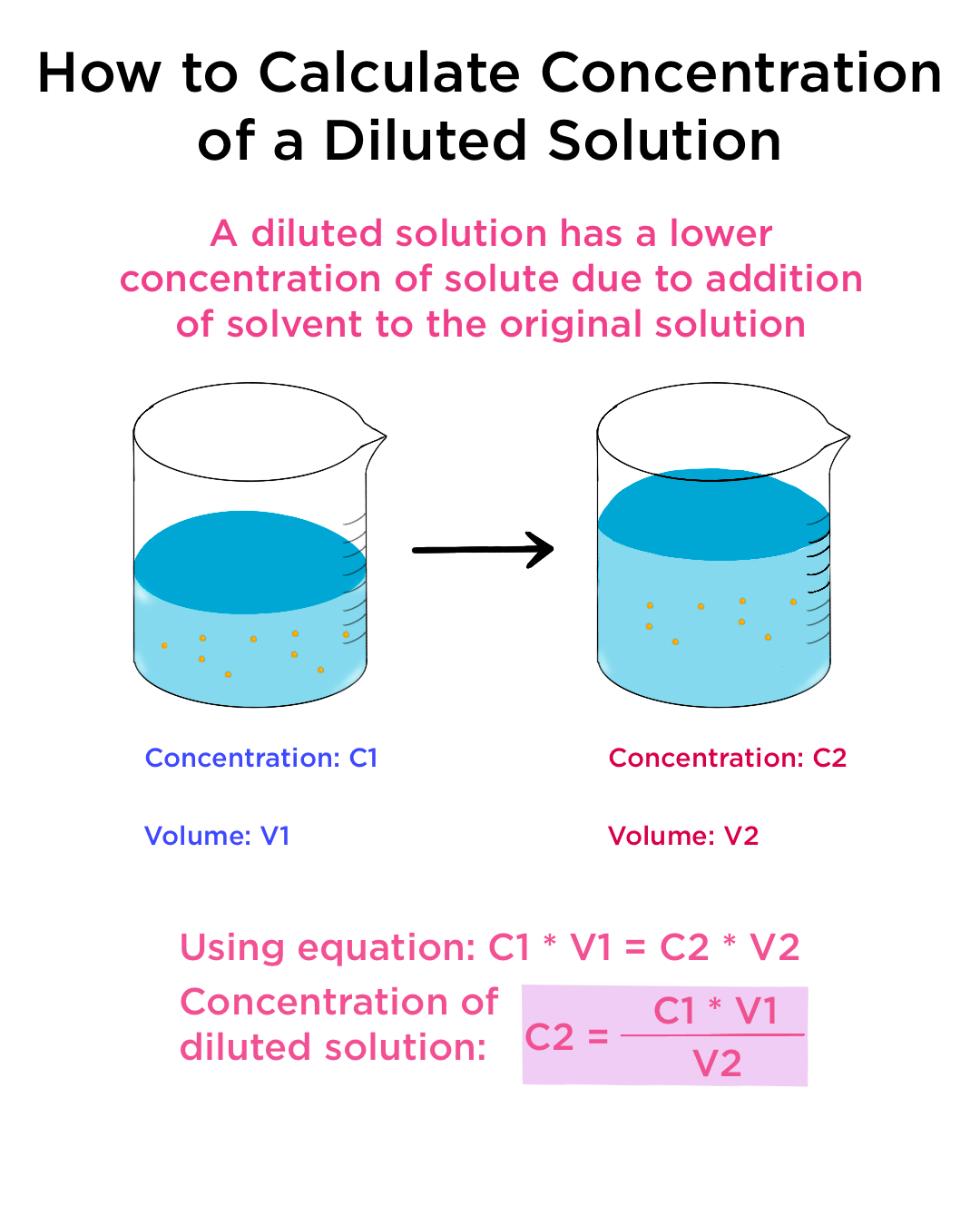
Dilute Solution More Concentrated . anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Often, a worker will need to change the concentration of a solution by changing the amount. we will begin our discussion of solution concentration with two related and relative terms: concentration refers to the amount of solute present in a given quantity (known volume) of a solution. Dilution is the process where the concentration of a solution is reduced by adding solvent. learn how to dilute and concentrate solutions. dilution is the process of making a concentrated solution less concentrated. by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration.
from www.expii.com
solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. dilution is the process of making a concentrated solution less concentrated. Often, a worker will need to change the concentration of a solution by changing the amount. we will begin our discussion of solution concentration with two related and relative terms: anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. concentration refers to the amount of solute present in a given quantity (known volume) of a solution. by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. learn how to dilute and concentrate solutions. Dilution is the process where the concentration of a solution is reduced by adding solvent.
Dilution of Solutions — Overview & Examples Expii
Dilute Solution More Concentrated Dilution is the process where the concentration of a solution is reduced by adding solvent. learn how to dilute and concentrate solutions. anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. Often, a worker will need to change the concentration of a solution by changing the amount. by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. dilution is the process of making a concentrated solution less concentrated. Dilution is the process where the concentration of a solution is reduced by adding solvent. concentration refers to the amount of solute present in a given quantity (known volume) of a solution. we will begin our discussion of solution concentration with two related and relative terms: solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute.
From www.youtube.com
Aqueous, Dilute And Concentrated Solution, General Science Lecture Dilute Solution More Concentrated learn how to dilute and concentrate solutions. dilution is the process of making a concentrated solution less concentrated. Dilution is the process where the concentration of a solution is reduced by adding solvent. Often, a worker will need to change the concentration of a solution by changing the amount. anyone who has made instant coffee or lemonade. Dilute Solution More Concentrated.
From www.slideserve.com
PPT Mixtures, Solutions, and Water PowerPoint Presentation, free Dilute Solution More Concentrated solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. concentration refers to the amount of solute present in a given quantity (known volume) of a solution. dilution is the process of making a concentrated solution less concentrated. Often, a worker will need to change the concentration of a solution. Dilute Solution More Concentrated.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Solution More Concentrated solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. we will begin our discussion of solution concentration with two related and relative terms: dilution is the process of making. Dilute Solution More Concentrated.
From www.showme.com
Concentrated vs. Diluted Solution Science ShowMe Dilute Solution More Concentrated Dilution is the process where the concentration of a solution is reduced by adding solvent. anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. we will begin our discussion of. Dilute Solution More Concentrated.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Solution More Concentrated Dilution is the process where the concentration of a solution is reduced by adding solvent. anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. Often, a worker will need to change the concentration of a solution by changing the amount. we will begin our discussion of solution concentration. Dilute Solution More Concentrated.
From www.geeksforgeeks.org
Concentration of Solution Definition, Formulas & Solved Examples Dilute Solution More Concentrated learn how to dilute and concentrate solutions. Dilution is the process where the concentration of a solution is reduced by adding solvent. we will begin our discussion of solution concentration with two related and relative terms: by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. anyone. Dilute Solution More Concentrated.
From www.slideserve.com
PPT Dilute and Concentrated Solutions PowerPoint Presentation, free Dilute Solution More Concentrated by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. learn how to dilute and concentrate solutions. anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. dilution is the process of making a concentrated solution less concentrated.. Dilute Solution More Concentrated.
From brainly.ph
What is the difference between a dilute solution and a concentrated Dilute Solution More Concentrated anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. we will begin our discussion of solution concentration with two related and relative terms: Often, a worker will need to change. Dilute Solution More Concentrated.
From www.slideserve.com
PPT Grade 7 Science PowerPoint Presentation, free download ID2229802 Dilute Solution More Concentrated we will begin our discussion of solution concentration with two related and relative terms: learn how to dilute and concentrate solutions. concentration refers to the amount of solute present in a given quantity (known volume) of a solution. Often, a worker will need to change the concentration of a solution by changing the amount. Dilution is the. Dilute Solution More Concentrated.
From www.youtube.com
Dilute and Concentrated Solution YouTube Dilute Solution More Concentrated by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Often, a worker will need to change the concentration of a solution by changing the amount. anyone who has made instant. Dilute Solution More Concentrated.
From www.slideserve.com
PPT What is diffusion? PowerPoint Presentation, free download ID Dilute Solution More Concentrated Dilution is the process where the concentration of a solution is reduced by adding solvent. anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. concentration refers to the amount of solute present in a given quantity (known volume) of a solution. solutions can be prepared by diluting. Dilute Solution More Concentrated.
From brainly.in
Difference between dilute and concentrated solution Brainly.in Dilute Solution More Concentrated Often, a worker will need to change the concentration of a solution by changing the amount. we will begin our discussion of solution concentration with two related and relative terms: solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. anyone who has made instant coffee or lemonade knows that. Dilute Solution More Concentrated.
From www.shutterstock.com
228 Dilute Solution Concentrated Solution Images, Stock Photos Dilute Solution More Concentrated we will begin our discussion of solution concentration with two related and relative terms: anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. Dilution is the process where the concentration of a solution is reduced by adding solvent. by adding solvent to a measured portion of a. Dilute Solution More Concentrated.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Solution More Concentrated learn how to dilute and concentrate solutions. Dilution is the process where the concentration of a solution is reduced by adding solvent. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. we will begin our discussion of solution concentration with two related and relative terms: dilution is the. Dilute Solution More Concentrated.
From www.slideshare.net
Chapter 16 solutions Dilute Solution More Concentrated solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. concentration refers to the amount of solute present in a given quantity (known volume) of a solution. Dilution is the process where the concentration of a solution is reduced by adding solvent. Often, a worker will need to change the concentration. Dilute Solution More Concentrated.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilute Solution More Concentrated learn how to dilute and concentrate solutions. we will begin our discussion of solution concentration with two related and relative terms: dilution is the process of making a concentrated solution less concentrated. by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. Often, a worker will need. Dilute Solution More Concentrated.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Solution More Concentrated Dilution is the process where the concentration of a solution is reduced by adding solvent. Often, a worker will need to change the concentration of a solution by changing the amount. anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. dilution is the process of making a concentrated. Dilute Solution More Concentrated.
From byjus.com
What is the difference between concentrated, aqueous and dilute solution? Dilute Solution More Concentrated anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. Often, a worker will need to change the concentration of a solution by changing the amount. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. learn how to dilute and. Dilute Solution More Concentrated.
From fphoto.photoshelter.com
science chemistry solubility dilution Fundamental Photographs The Dilute Solution More Concentrated concentration refers to the amount of solute present in a given quantity (known volume) of a solution. we will begin our discussion of solution concentration with two related and relative terms: solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. by adding solvent to a measured portion of. Dilute Solution More Concentrated.
From www.youtube.com
What is Dilute Solution? Chemistry YouTube Dilute Solution More Concentrated concentration refers to the amount of solute present in a given quantity (known volume) of a solution. learn how to dilute and concentrate solutions. Often, a worker will need to change the concentration of a solution by changing the amount. dilution is the process of making a concentrated solution less concentrated. we will begin our discussion. Dilute Solution More Concentrated.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Solution More Concentrated by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. Dilution is the process where the concentration of a solution is reduced by adding solvent. Often, a worker will need to change the concentration of a solution by changing the amount. dilution is the process of making a concentrated. Dilute Solution More Concentrated.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilute Solution More Concentrated Often, a worker will need to change the concentration of a solution by changing the amount. by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. we will begin our discussion of solution concentration with two related and relative terms: dilution is the process of making a concentrated. Dilute Solution More Concentrated.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilute Solution More Concentrated anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. Dilution is the process where the concentration of a solution is reduced by adding solvent. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. we will begin our discussion of. Dilute Solution More Concentrated.
From www.slideserve.com
PPT Section 6.2—Concentration PowerPoint Presentation, free download Dilute Solution More Concentrated concentration refers to the amount of solute present in a given quantity (known volume) of a solution. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. we will begin our discussion of solution concentration with two related and relative terms: learn how to dilute and concentrate solutions. . Dilute Solution More Concentrated.
From www.aiophotoz.com
What Is The Difference Between Dilute And Concentrated Solution Dilute Solution More Concentrated learn how to dilute and concentrate solutions. by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. we will begin our discussion of solution concentration with two related and relative terms: anyone who has made instant coffee or lemonade knows that too much powder gives a strongly. Dilute Solution More Concentrated.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Dilute Solution More Concentrated Dilution is the process where the concentration of a solution is reduced by adding solvent. concentration refers to the amount of solute present in a given quantity (known volume) of a solution. learn how to dilute and concentrate solutions. by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular. Dilute Solution More Concentrated.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilute Solution More Concentrated Dilution is the process where the concentration of a solution is reduced by adding solvent. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. we will begin our discussion of. Dilute Solution More Concentrated.
From www.quora.com
What is the difference between a concentrated (saturated) solution and Dilute Solution More Concentrated Dilution is the process where the concentration of a solution is reduced by adding solvent. learn how to dilute and concentrate solutions. concentration refers to the amount of solute present in a given quantity (known volume) of a solution. Often, a worker will need to change the concentration of a solution by changing the amount. dilution is. Dilute Solution More Concentrated.
From slidetodoc.com
WATER AND SOLUTIONS A solution is a mixture Dilute Solution More Concentrated solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. Often, a worker will need to change the concentration of a solution by changing the amount. we will begin our discussion of solution concentration with two related and relative terms: Dilution is the process where the concentration of a solution is. Dilute Solution More Concentrated.
From stock.adobe.com
A concentrated solution is one that has a relatively large amount of Dilute Solution More Concentrated anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. Dilution is the process where the concentration of a solution is reduced by adding solvent. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. dilution is the process of making. Dilute Solution More Concentrated.
From www.youtube.com
Dilute, Concentrated and saturated solution YouTube Dilute Solution More Concentrated anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. we will begin our discussion of solution concentration with two related and relative terms: by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. concentration refers to the. Dilute Solution More Concentrated.
From www.youtube.com
3. Strong, weak, dilute and concentrated acids (HSC chemistry) YouTube Dilute Solution More Concentrated by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. solutions can be prepared by diluting a more concentrated solution rather than starting with the pure solute. concentration refers to the amount of solute present in a given quantity (known volume) of a solution. anyone who has. Dilute Solution More Concentrated.
From ar.inspiredpencil.com
Concentrated Vs Dilute Solutions Dilute Solution More Concentrated anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. Dilution is the process where the concentration of a solution is reduced by adding solvent. by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. concentration refers to the. Dilute Solution More Concentrated.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution More Concentrated we will begin our discussion of solution concentration with two related and relative terms: by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. anyone who has made instant coffee or lemonade knows that too much powder gives a strongly flavored, highly concentrated. Dilution is the process where. Dilute Solution More Concentrated.
From www.yaclass.in
Types of solutions Concentrated and dilute solutions — lesson. Science Dilute Solution More Concentrated by adding solvent to a measured portion of a more concentrated stock solution, we can achieve a particular concentration. dilution is the process of making a concentrated solution less concentrated. we will begin our discussion of solution concentration with two related and relative terms: Often, a worker will need to change the concentration of a solution by. Dilute Solution More Concentrated.